
Draw structure of \[HCl{O_4}\].
Answer
593.1k+ views
Hint:A structural formula of a compound represents the structure of a particular compound. Lewis structures are formulated from the concept of the electron dot diagram. In this structure, lines are added between the atoms to represent the shared pair in a particular chemical bond.
Complete step by step answer:
We know that Hydrogen atom has only one valence electron, chlorine has seven valence electrons and oxygen atom has six valence electrons. We can draw the Lewis dot structure of \[{\text{HCl}}{{\text{O}}_{\text{4}}}\] as follows.
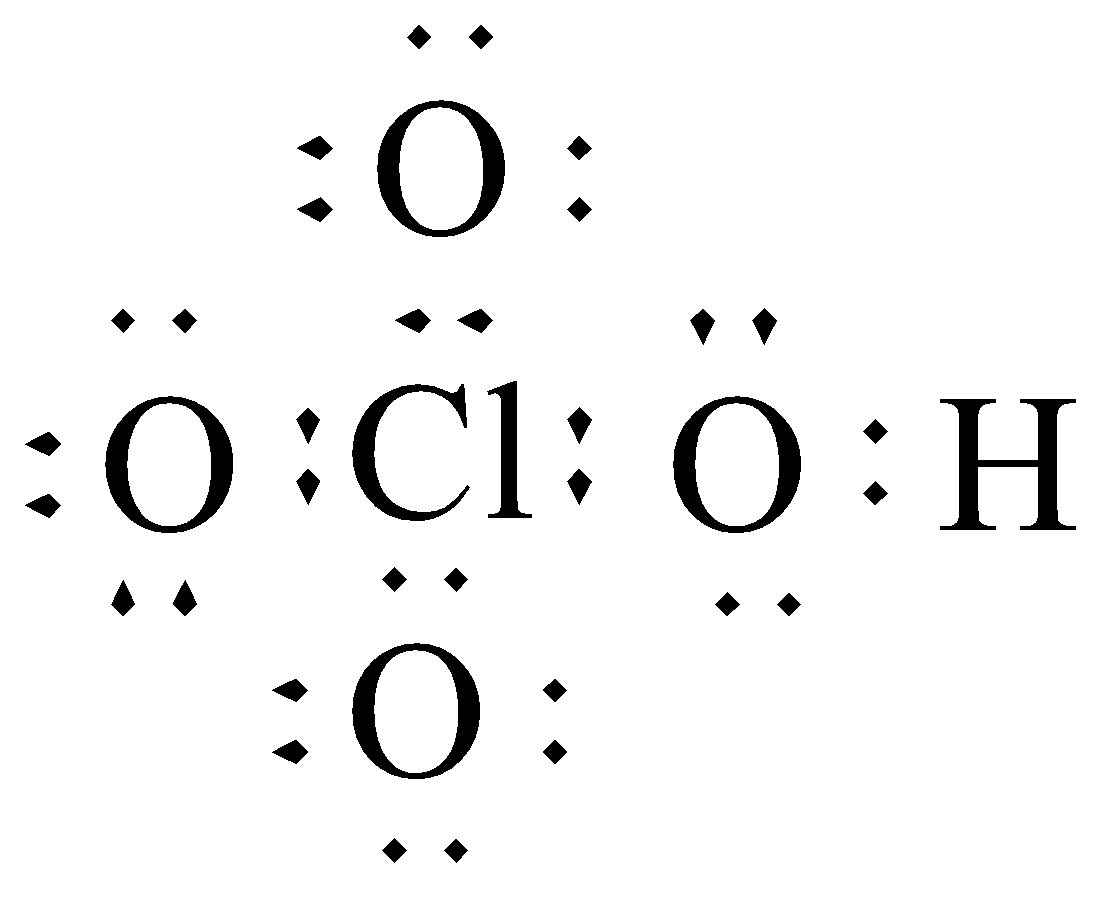
Lewis-dot structure of \[{\text{HCl}}{{\text{O}}_{\text{4}}}\]
And we can draw the structural formula of this compound as follows.
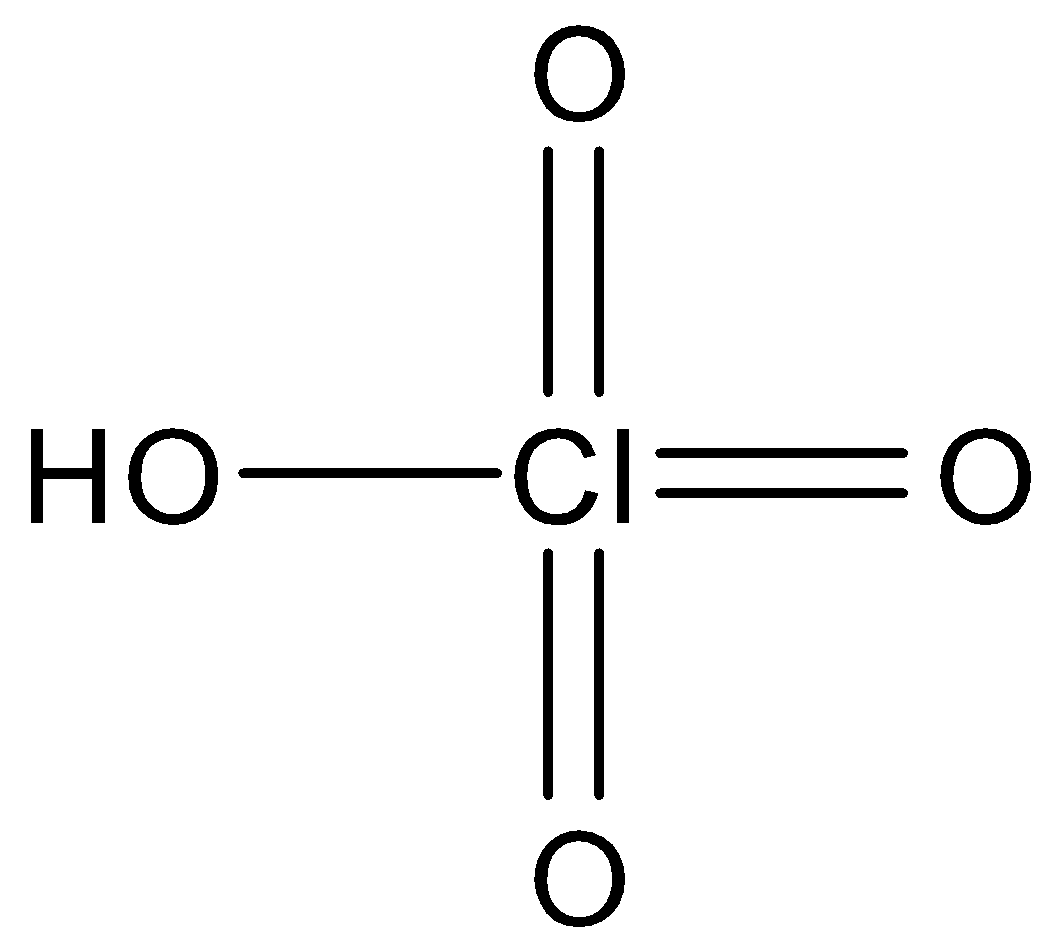
Additional information:
Perchloric acid is usually found as an aqueous solution. It is a colourless compound and it is a stronger acid than nitric acid and sulfuric acid.
We can prepare at industries by two methods. In the traditional method we make use of the high aqueous solubility of sodium perchlorate \[\left( {{\text{NaCl}}{{\text{O}}_{\text{4}}}} \right)\]. Treating this solution with the hydrochloric acid \[\left( {{\text{HCl}}} \right)\]produces perchloric acid by the precipitation of solid sodium chloride. We can write the reaction of this preparation follows:
\[{\text{NaCl}}{{\text{O}}_{\text{4}}} + {\text{HCl}} \to {\text{NaCl}} + {\text{HCl}}{{\text{O}}_{\text{4}}}\]
Perchloric acid has various uses. It is used in making explosives, in the separation of sodium and potassium, it is used as an oxidizer, it is used in the rocket fuel ,used in plating of metals, used as catalyst, used for electropolishing of molybdenum, used as a reagent for determining the 1H-Benzotriazole etc.
Note:
\[{\text{HCl}}{{\text{O}}_{\text{4}}}\] is commonly called perchloric acid. An alternate name for this compound is hyperchloric acid. According to the IUPAC nomenclature \[{\text{HCl}}{{\text{O}}_{\text{4}}}\] is named as chloric (VII) acid.
Complete step by step answer:
We know that Hydrogen atom has only one valence electron, chlorine has seven valence electrons and oxygen atom has six valence electrons. We can draw the Lewis dot structure of \[{\text{HCl}}{{\text{O}}_{\text{4}}}\] as follows.
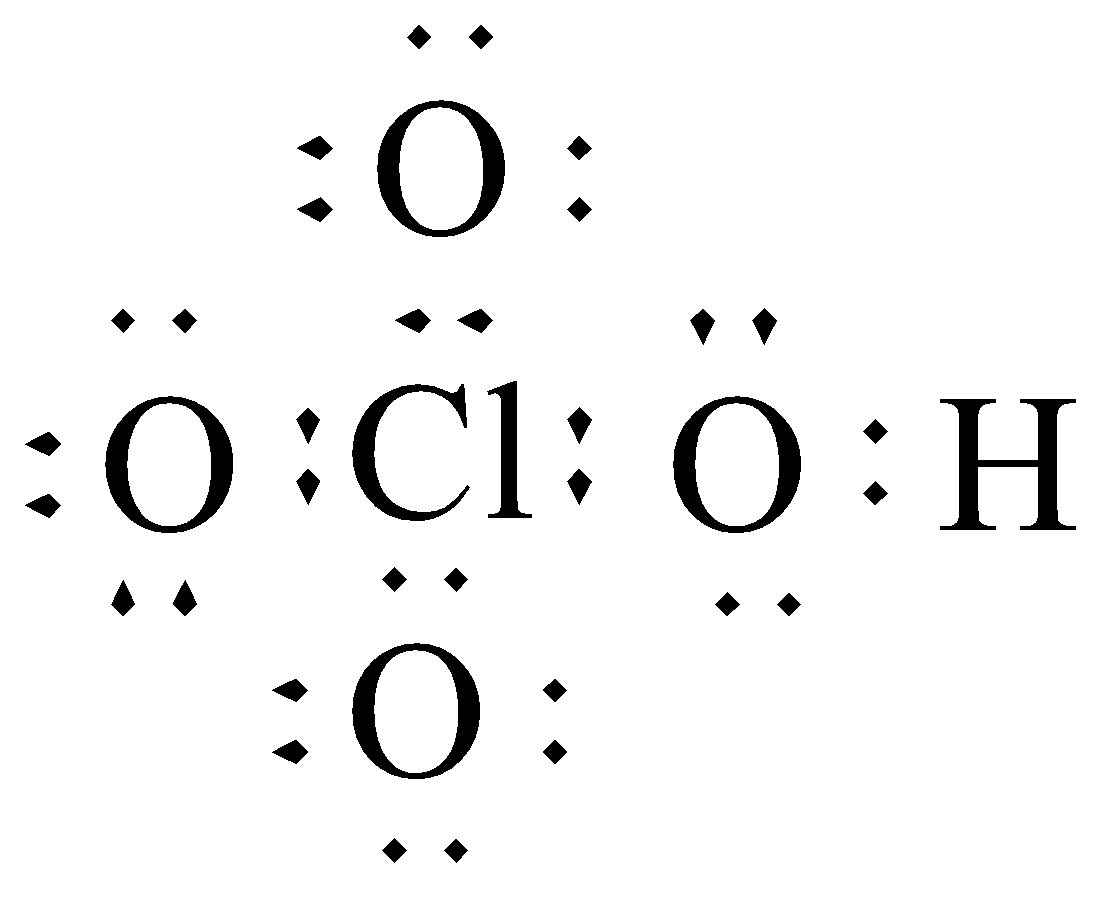
Lewis-dot structure of \[{\text{HCl}}{{\text{O}}_{\text{4}}}\]
And we can draw the structural formula of this compound as follows.

Additional information:
Perchloric acid is usually found as an aqueous solution. It is a colourless compound and it is a stronger acid than nitric acid and sulfuric acid.
We can prepare at industries by two methods. In the traditional method we make use of the high aqueous solubility of sodium perchlorate \[\left( {{\text{NaCl}}{{\text{O}}_{\text{4}}}} \right)\]. Treating this solution with the hydrochloric acid \[\left( {{\text{HCl}}} \right)\]produces perchloric acid by the precipitation of solid sodium chloride. We can write the reaction of this preparation follows:
\[{\text{NaCl}}{{\text{O}}_{\text{4}}} + {\text{HCl}} \to {\text{NaCl}} + {\text{HCl}}{{\text{O}}_{\text{4}}}\]
Perchloric acid has various uses. It is used in making explosives, in the separation of sodium and potassium, it is used as an oxidizer, it is used in the rocket fuel ,used in plating of metals, used as catalyst, used for electropolishing of molybdenum, used as a reagent for determining the 1H-Benzotriazole etc.
Note:
\[{\text{HCl}}{{\text{O}}_{\text{4}}}\] is commonly called perchloric acid. An alternate name for this compound is hyperchloric acid. According to the IUPAC nomenclature \[{\text{HCl}}{{\text{O}}_{\text{4}}}\] is named as chloric (VII) acid.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




