
Draw Fischer projection of D-Glucose and L-Glucose.
Answer
540.9k+ views
Hint: Before solving this question, we should first know what is the Fischer projection of D-Glucose and L-Glucose and then we can draw it. Fischer projection is a representation of a three-dimensional organic molecule in two- dimensions with the help of projection.
Complete step-by-step answer:
In 1891, Emil Fischer came up with the Fischer projection. Initially, It was introduced for the illustration of carbohydrates and it was used by chemists in biochemistry and organic chemistry. In non-carbohydrates, Fischer projections are not used because these drawings are ambiguous.
They are commonly used to represent monosaccharides in biochemistry and organic chemistry. A Fischer projection is mainly used to distinguish between L- and D- molecules.
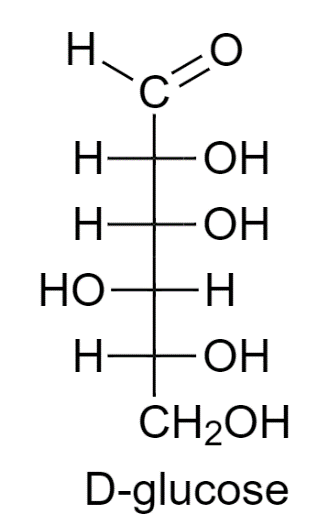
Glucose is a sugar molecule that can be found in two states D- Glucose or L- Glucose.
D- Glucose is present in a huge amount in nature. It is used to rotate the plane-polarized light in a clockwise direction. The –OH group of the main carbon is on the left side whereas the other –OH groups are on the right side.
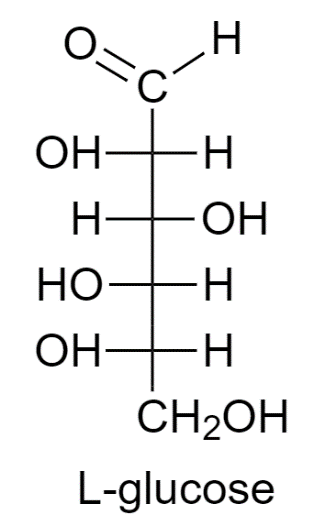
L- Glucose is present in less amount in nature. It is used to rotate the plane-polarized light in an anticlockwise direction. The –OH group of the main carbon is on the right side whereas the other –OH groups are on the left side.
Note:
There are two kinds of isomerism, they are structural isomerism and stereoisomerism. The isomers that have the same chemical structure but their mirror image cannot be superimposed on each other. D and L come under the category of stereoisomers.
Complete step-by-step answer:
In 1891, Emil Fischer came up with the Fischer projection. Initially, It was introduced for the illustration of carbohydrates and it was used by chemists in biochemistry and organic chemistry. In non-carbohydrates, Fischer projections are not used because these drawings are ambiguous.
They are commonly used to represent monosaccharides in biochemistry and organic chemistry. A Fischer projection is mainly used to distinguish between L- and D- molecules.

Glucose is a sugar molecule that can be found in two states D- Glucose or L- Glucose.
D- Glucose is present in a huge amount in nature. It is used to rotate the plane-polarized light in a clockwise direction. The –OH group of the main carbon is on the left side whereas the other –OH groups are on the right side.

L- Glucose is present in less amount in nature. It is used to rotate the plane-polarized light in an anticlockwise direction. The –OH group of the main carbon is on the right side whereas the other –OH groups are on the left side.
Note:
There are two kinds of isomerism, they are structural isomerism and stereoisomerism. The isomers that have the same chemical structure but their mirror image cannot be superimposed on each other. D and L come under the category of stereoisomers.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




