
Draw all the isomers (geometrical and optical) of:
(1) \[{{\left[ CoC{{l}_{2}}{{\left( en \right)}_{2}} \right]}^{+}}\]
(2) \[{{\left[ Co\left( N{{H}_{3}} \right)Cl{{\left( en \right)}_{2}} \right]}^{2+}}~\]
(3) \[{{\left[ Co{{\left( N{{H}_{3}} \right)}_{2}}C{{l}_{2}}\left( en \right) \right]}^{+}}\]
Answer
573.6k+ views
Hint: Geometrical and optical isomers are subdivisions of stereoisomerism as it deals with the stereochemistry of the molecule. In other words, it takes in account the spatial arrangement of a molecule or ion, as well as the superimposable or non-superimposable characteristics of mirror images.
Complete step by step answer:
The molecules that have the same molecular formula, but the ones who have a different arrangement of the atoms of the molecule in space are termed as Isomers. This definition excludes any different type of arrangements which are simply because of the molecular rotation as a whole, or rotating about specific bonds. The atoms in which making up the numerous isomers are connected in a different order, the phenomena is known as structural isomerism.
In stereoisomerism, the atoms which are making up the isomers are connected in the same order, but they still manage to have a different type of spatial arrangement. Geometric isomerism is a type of stereoisomerism.
The geometrical isomers are those isomers that occur where we have restricted rotation somewhere in a molecule. Some common examples usually include the carbon-carbon double bonds. When two carbons are connected by a double bond, they have restricted rotation as the bond will break if they tried to undergo rotation. Unlike a single bond, where rotation is much easier.
There are two subdivision of geometrical isomerism, cis and trans isomerism.
Trans isomerism is the type where the functional groups are present at different sides of the double bond. And cis isomerism is where the functional group is present at the same side of the double bond.
Geometrical isomerism of \[{{\left[ CoC{{l}_{2}}{{\left( en \right)}_{2}} \right]}^{+}}\] are
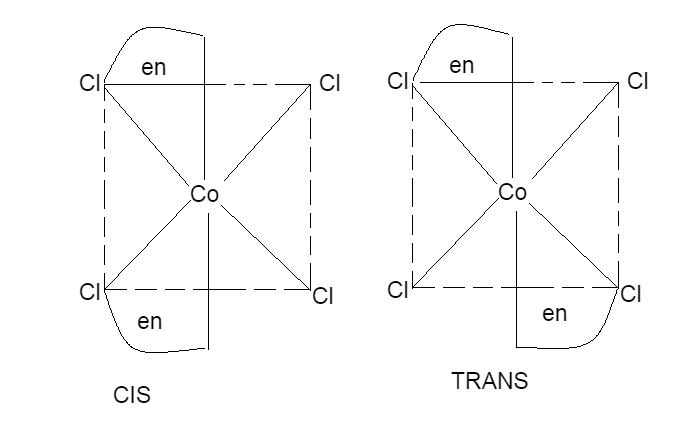
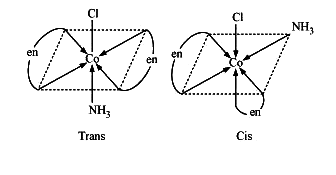
The geometrical isomers of \[{{\left[ Co\left( N{{H}_{3}} \right)Cl{{\left( en \right)}_{2}} \right]}^{2+}}~\] are,
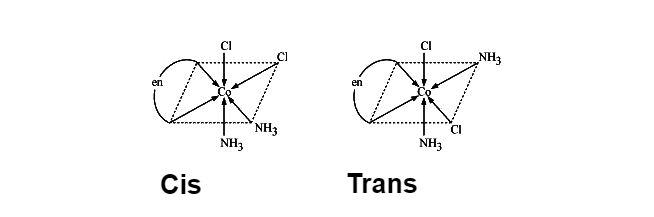
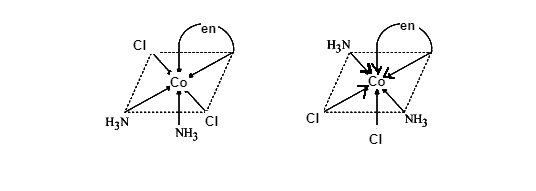
Geometrical isomers of \[{{\left[ Co{{\left( N{{H}_{3}} \right)}_{2}}C{{l}_{2}}\left( en \right) \right]}^{+}}\],
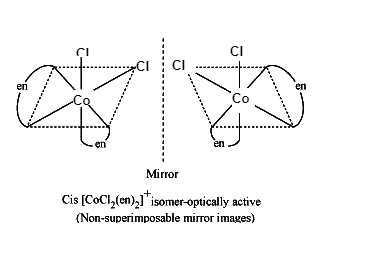
While on the other hand Optical isomers are two compounds in which the same number and kinds of atoms are present, and bonds (as in the connectivity between the atoms involved in the bonding is the same), and they also have different spatial arrangements of the atoms, but optical isomers have non-superimposable mirror images. Each of these mirror image structures is termed as an enantiomer. Ions or Molecules that exist as optical isomers are termed as chiral ions or molecules.
Optical isomers of \[{{\left[ CoC{{l}_{2}}{{\left( en \right)}_{2}} \right]}^{+}}\],
Optical isomers of \[{{\left[ Co\left( N{{H}_{3}} \right)Cl{{\left( en \right)}_{2}} \right]}^{2+}}~\]
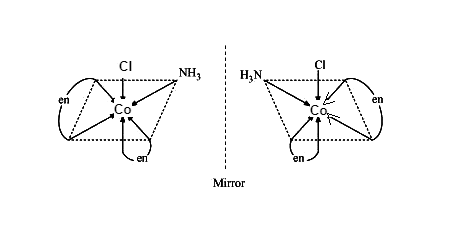
Optical isomers of \[{{\left[ Co{{\left( N{{H}_{3}} \right)}_{2}}C{{l}_{2}}\left( en \right) \right]}^{+}}\]
Note: Structural isomerism is not a form of stereoisomerism, it is a different type of isomerism and should be confused with stereoisomerism. The definition of isomers excludes any different type of arrangements which are simply because of the molecular rotation as a whole, or rotating about specific bonds
Complete step by step answer:
The molecules that have the same molecular formula, but the ones who have a different arrangement of the atoms of the molecule in space are termed as Isomers. This definition excludes any different type of arrangements which are simply because of the molecular rotation as a whole, or rotating about specific bonds. The atoms in which making up the numerous isomers are connected in a different order, the phenomena is known as structural isomerism.
In stereoisomerism, the atoms which are making up the isomers are connected in the same order, but they still manage to have a different type of spatial arrangement. Geometric isomerism is a type of stereoisomerism.
The geometrical isomers are those isomers that occur where we have restricted rotation somewhere in a molecule. Some common examples usually include the carbon-carbon double bonds. When two carbons are connected by a double bond, they have restricted rotation as the bond will break if they tried to undergo rotation. Unlike a single bond, where rotation is much easier.
There are two subdivision of geometrical isomerism, cis and trans isomerism.
Trans isomerism is the type where the functional groups are present at different sides of the double bond. And cis isomerism is where the functional group is present at the same side of the double bond.
Geometrical isomerism of \[{{\left[ CoC{{l}_{2}}{{\left( en \right)}_{2}} \right]}^{+}}\] are

The geometrical isomers of \[{{\left[ Co\left( N{{H}_{3}} \right)Cl{{\left( en \right)}_{2}} \right]}^{2+}}~\] are,

Geometrical isomers of \[{{\left[ Co{{\left( N{{H}_{3}} \right)}_{2}}C{{l}_{2}}\left( en \right) \right]}^{+}}\],

While on the other hand Optical isomers are two compounds in which the same number and kinds of atoms are present, and bonds (as in the connectivity between the atoms involved in the bonding is the same), and they also have different spatial arrangements of the atoms, but optical isomers have non-superimposable mirror images. Each of these mirror image structures is termed as an enantiomer. Ions or Molecules that exist as optical isomers are termed as chiral ions or molecules.
Optical isomers of \[{{\left[ CoC{{l}_{2}}{{\left( en \right)}_{2}} \right]}^{+}}\],

Optical isomers of \[{{\left[ Co\left( N{{H}_{3}} \right)Cl{{\left( en \right)}_{2}} \right]}^{2+}}~\]

Optical isomers of \[{{\left[ Co{{\left( N{{H}_{3}} \right)}_{2}}C{{l}_{2}}\left( en \right) \right]}^{+}}\]

Note: Structural isomerism is not a form of stereoisomerism, it is a different type of isomerism and should be confused with stereoisomerism. The definition of isomers excludes any different type of arrangements which are simply because of the molecular rotation as a whole, or rotating about specific bonds
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




