
Draw all of the isomers of both tetrahedral and square planar complexes which have two unidentate ligands of type A and two unidentate ligands of type B.
Answer
509.1k+ views
Hint: When two or more complexes have same atoms, same numbers of same atoms, but the complexes vary in the spatial arrangements is said to be isomers. The phenomena of a complex having various isomers are called isomerism. The word isomer is derived from the Greek language which in English means as iso is same and mers means parts.
Complete answer:
When a complex having one central metal atom and more than one type of ligand tends to have different arrangements, this leads to isomerisation. Isomers are generally classified into two types namely (a) structural isomers and (b) stereoisomers.
Structural isomers are those particular types of isomers which are due to the variation of the structure of the complex. Structural Isomers are further classified into four types which are as follows:- (i) Ionisation Isomers, this type of isomerism arises due to different ions or molecules present in the coordination sphere and counter ion but are exchangeable. (ii) Hydrate Isomers, are those particular types of isomers, in which the water molecule will replace the counter ion position. (iii) Linkage Isomerism, are those isomers in which one of the ligands is ambidentate. (iv) Coordination Isomerism arises due to difference in the ligands. (v) Position Isomerism occurs due to bridging of ligands. (vi) Polymerisation Isomerism, in which the central metal atom’s coordination sphere breaks into two having the same metal but different ligands that are already present in the coordination sphere.
Stereo Isomers are those isomers which arise due to differences in the arrangements of ligands. They are of two types as follows:- (i) Geometrical Isomerism, are the spatial arrangements of the atoms. (ii)Optical Isomerism rotates the plane polarised light.
All of the isomers of both tetrahedral and square planar complexes which have two unidentate ligands of type A and two unidentate ligands of type B are as follows
In the case of tetrahedral no geometrical isomerism exists because all the four positions are equivalent.
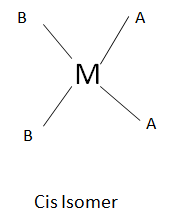
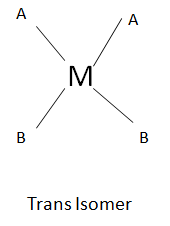
In case of square planar, following geometrical isomers exists
Note:
Isomerism leads to differences in various properties like in boiling point, reactivity, etc. However due to isomerisation, the toxicity of the complex also varies like L-Dopa is used to cure Parkinson disease but D-Dopa is toxic not only to humans. The tetrahedral no geometrical isomerism exists because all the four positions are equivalent whereas in case of square planar two stereo isomers exists namely Cis and Trans.
Complete answer:
When a complex having one central metal atom and more than one type of ligand tends to have different arrangements, this leads to isomerisation. Isomers are generally classified into two types namely (a) structural isomers and (b) stereoisomers.
Structural isomers are those particular types of isomers which are due to the variation of the structure of the complex. Structural Isomers are further classified into four types which are as follows:- (i) Ionisation Isomers, this type of isomerism arises due to different ions or molecules present in the coordination sphere and counter ion but are exchangeable. (ii) Hydrate Isomers, are those particular types of isomers, in which the water molecule will replace the counter ion position. (iii) Linkage Isomerism, are those isomers in which one of the ligands is ambidentate. (iv) Coordination Isomerism arises due to difference in the ligands. (v) Position Isomerism occurs due to bridging of ligands. (vi) Polymerisation Isomerism, in which the central metal atom’s coordination sphere breaks into two having the same metal but different ligands that are already present in the coordination sphere.
Stereo Isomers are those isomers which arise due to differences in the arrangements of ligands. They are of two types as follows:- (i) Geometrical Isomerism, are the spatial arrangements of the atoms. (ii)Optical Isomerism rotates the plane polarised light.
All of the isomers of both tetrahedral and square planar complexes which have two unidentate ligands of type A and two unidentate ligands of type B are as follows
In the case of tetrahedral no geometrical isomerism exists because all the four positions are equivalent.
In case of square planar, following geometrical isomers exists


Note:
Isomerism leads to differences in various properties like in boiling point, reactivity, etc. However due to isomerisation, the toxicity of the complex also varies like L-Dopa is used to cure Parkinson disease but D-Dopa is toxic not only to humans. The tetrahedral no geometrical isomerism exists because all the four positions are equivalent whereas in case of square planar two stereo isomers exists namely Cis and Trans.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




