
Draw a diagram of two dimensional square close packing.
Answer
540k+ views
Hint: Crystalline solids display an ordinary and rehashing example of constituent particles. The diagrammatic portrayal of three-dimensional arrangements of constituent particles in a crystal, in which every molecule is portrayed as a point in space is known as a crystal lattice. In a crystal lattice, the molecules are firmly packed leaving almost no space between them.
Complete step by step answer:
In two-dimensional close packing, rows of closed packed spheres are stacked to obtain a two-dimensional pattern. This stacking is done in two ways: Square close packing: The second row can be placed exactly below the first row in a close packing.
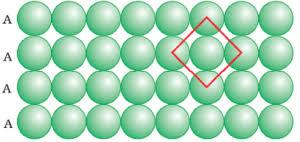
The second row can be placed exactly below the first row in a close packing. Thus, if we call the first row as “A” type row, the second row being arranged exactly the same as the first one, is also of “A” type. In such an arrangement each sphere is in contact with four other spheres. Hence, it has a coordination number equal to four. We observe that if the centres of the four immediate neighbouring spheres are joined, a square is formed. This type of packing in crystalline solids is known as square close packing in two dimensions.
Note: Near crystal packing refers to the effective arrangement of constituent particles in a crystal lattice in vacuum. We have to assume that all particles (atoms, molecules and ions) are of the same spherical solid form to grasp this set more precisely.
Complete step by step answer:
In two-dimensional close packing, rows of closed packed spheres are stacked to obtain a two-dimensional pattern. This stacking is done in two ways: Square close packing: The second row can be placed exactly below the first row in a close packing.

The second row can be placed exactly below the first row in a close packing. Thus, if we call the first row as “A” type row, the second row being arranged exactly the same as the first one, is also of “A” type. In such an arrangement each sphere is in contact with four other spheres. Hence, it has a coordination number equal to four. We observe that if the centres of the four immediate neighbouring spheres are joined, a square is formed. This type of packing in crystalline solids is known as square close packing in two dimensions.
Note: Near crystal packing refers to the effective arrangement of constituent particles in a crystal lattice in vacuum. We have to assume that all particles (atoms, molecules and ions) are of the same spherical solid form to grasp this set more precisely.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




