
Dow’s process is used for the preparation of which of the following?
(A) Esters
(B) Ethers
(C) Phenols
(D) Alcohols
Answer
571.8k+ views
Hint: Generally, the product we obtain from Dow’s process is mildly acidic in nature. The reaction involves the formation of benzyne as an intermediate. It is a substitution reaction.
Complete Solution :
We can prepare phenols from Dow’s process. Let’s see basic information about Dow’s process.
- In this method chlorobenzene (which is an example of haloarenes which is formed by monosubstitution of the benzene ring) is heated with aqueous NaOH at about 623K and 300 atm pressures to form sodium phenoxide, which is further acidified by dilute acid like HCl to form phenol.
Let’s understand the mechanism of this process.
- Step 1:
As we can see the base removes hydrogen from the benzene ring, in the form of ${H^ + }$ ion further to form carbanion. Then, chlorine ion leaves to form a triple bond in the ring and that species is called benzyne. Benzyne is very unstable. So it is a highly reactive species.
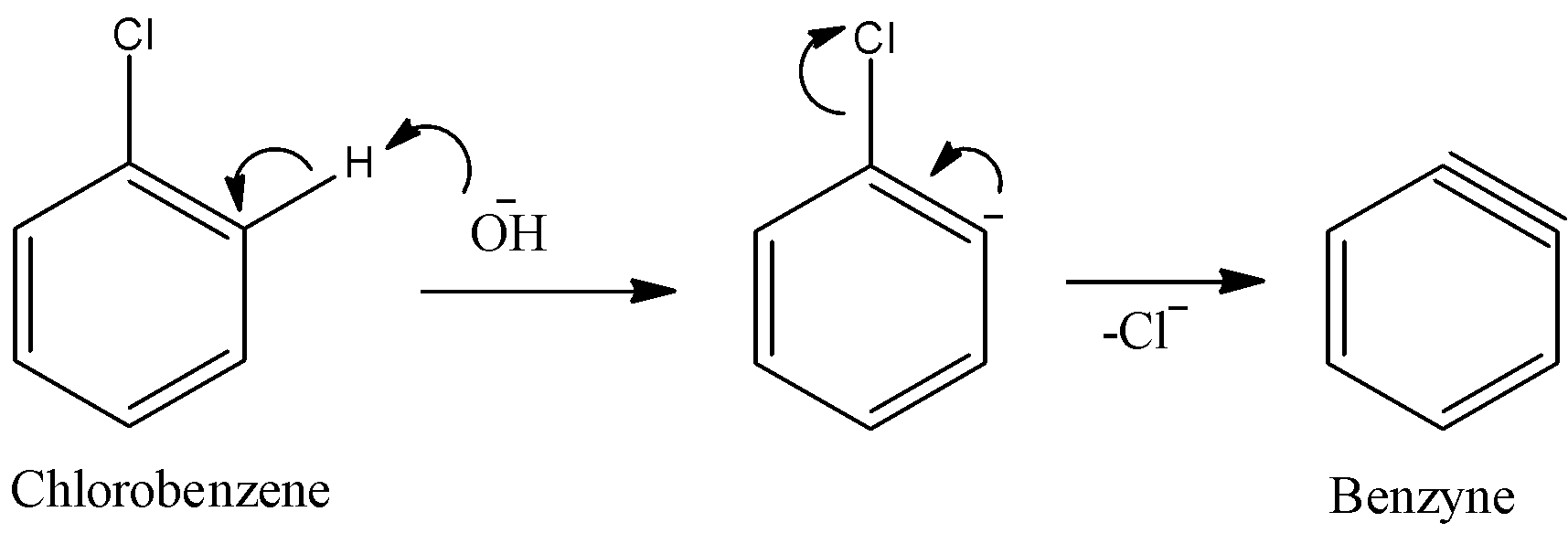
- Step 2:
Now, benzyne gets attacked by nucleophile $O{H^ - }$ to form another intermediate which is a carbanion which subsequently forms phenol upon reaction with proton. Thus, we obtain phenol as a final product in this reaction.
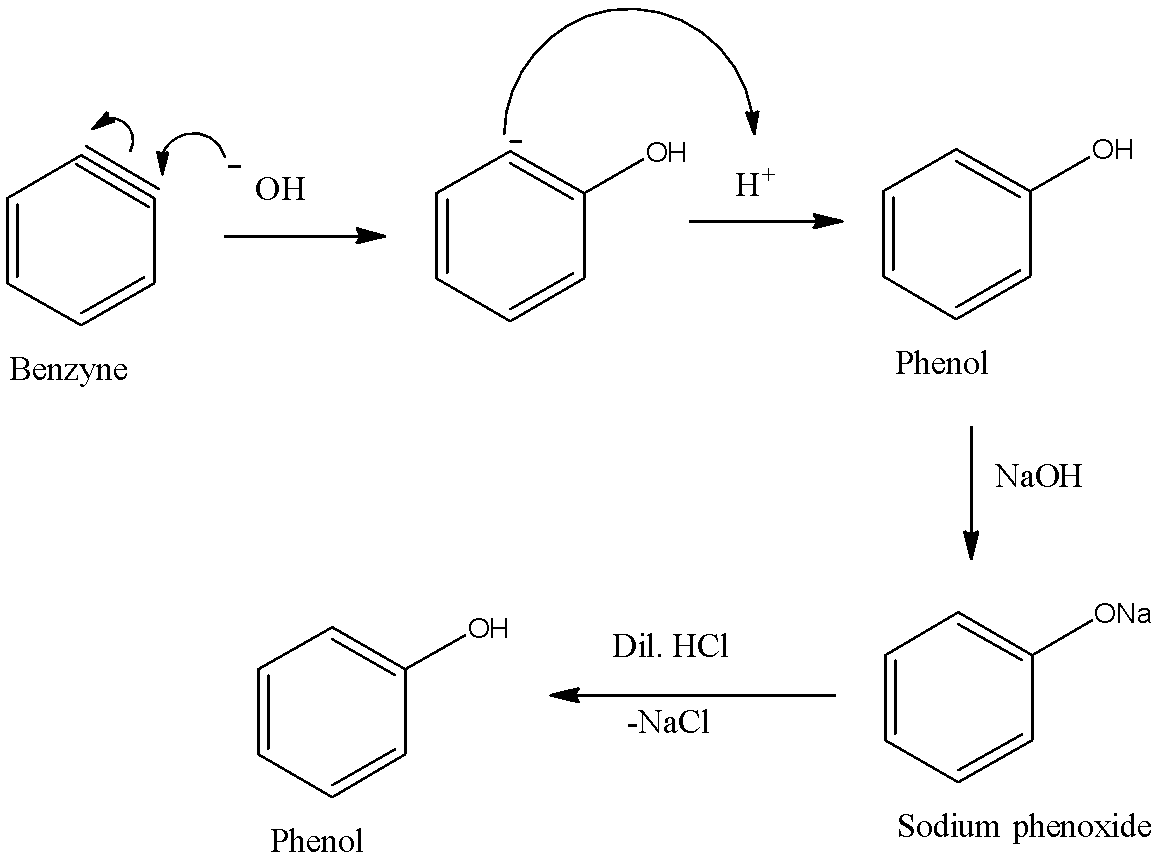
Therefore, Dow’s process is used for the preparation of phenols.
So, the correct answer is “Option C”.
Note: Note that here direct substitution of chlorine is not done by hydroxyl group. Actually removal of chlorine and proton generates benzyne and that is very reactive and it reacts with the nucleophile hydroxyl ion to give phenol. Presence of a halogen-aromatic carbon bond is necessary for the reactants in order to undergo Dow’s process.
Complete Solution :
We can prepare phenols from Dow’s process. Let’s see basic information about Dow’s process.
- In this method chlorobenzene (which is an example of haloarenes which is formed by monosubstitution of the benzene ring) is heated with aqueous NaOH at about 623K and 300 atm pressures to form sodium phenoxide, which is further acidified by dilute acid like HCl to form phenol.
Let’s understand the mechanism of this process.
- Step 1:
As we can see the base removes hydrogen from the benzene ring, in the form of ${H^ + }$ ion further to form carbanion. Then, chlorine ion leaves to form a triple bond in the ring and that species is called benzyne. Benzyne is very unstable. So it is a highly reactive species.

- Step 2:
Now, benzyne gets attacked by nucleophile $O{H^ - }$ to form another intermediate which is a carbanion which subsequently forms phenol upon reaction with proton. Thus, we obtain phenol as a final product in this reaction.

Therefore, Dow’s process is used for the preparation of phenols.
So, the correct answer is “Option C”.
Note: Note that here direct substitution of chlorine is not done by hydroxyl group. Actually removal of chlorine and proton generates benzyne and that is very reactive and it reacts with the nucleophile hydroxyl ion to give phenol. Presence of a halogen-aromatic carbon bond is necessary for the reactants in order to undergo Dow’s process.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




