
How does the structure of benzene differ from the cyclohexane structure?
Answer
552.6k+ views
Hint:The structure of benzene and cyclohexane has molecular formula as ${C_6}{H_6}$ and ${C_6}{H_{12}}$ respectively. Both the structures have six carbon atoms. One can think about the possible structure based upon the valency of the carbon atom and the number of hydrogen atoms present on each carbon in both structures.
Complete step by step answer:
1) First of all, we will see the structures of benzene and cyclohexane and analyze their structures with respect to each other. The structure of benzene has molecular formula as ${C_6}{H_6}$ and the structure representation will be as below,
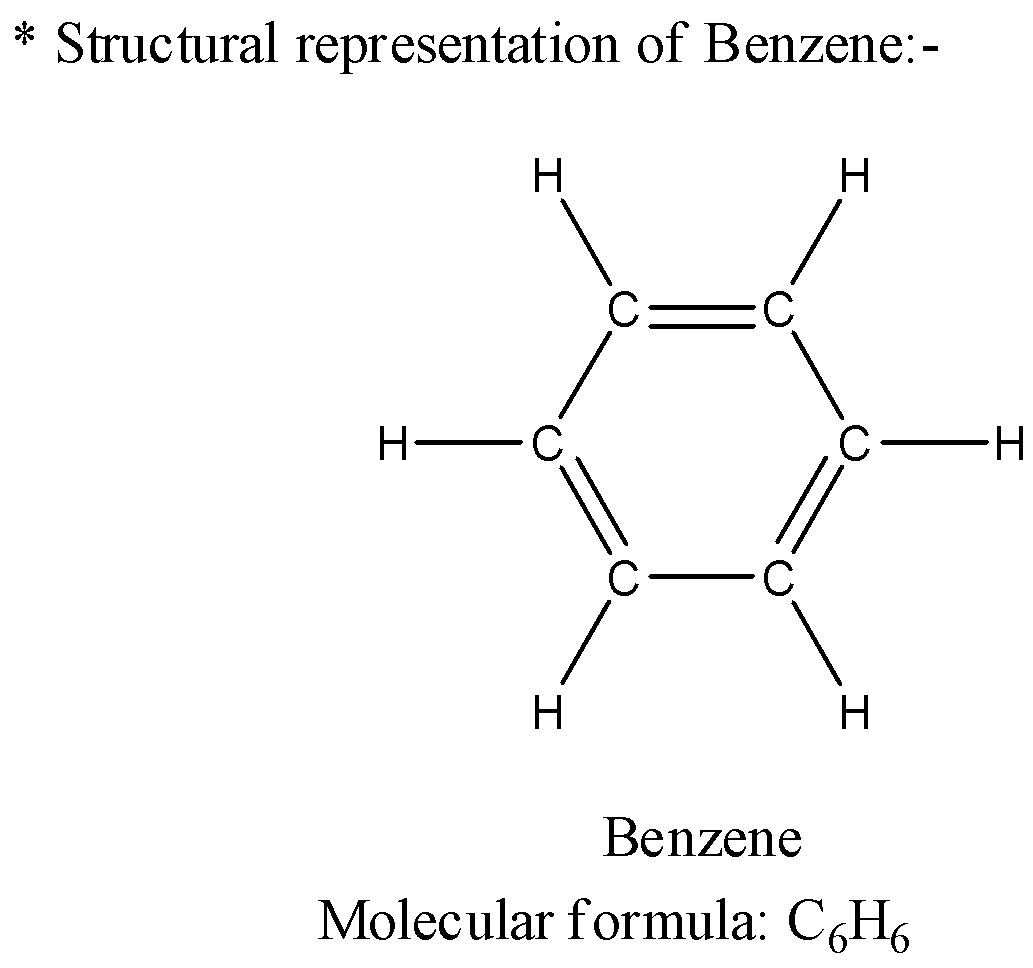
2) Now let us see the structure of the cyclohexane molecule which has molecular formula as ${C_6}{H_6}$ and the structure will be as below,
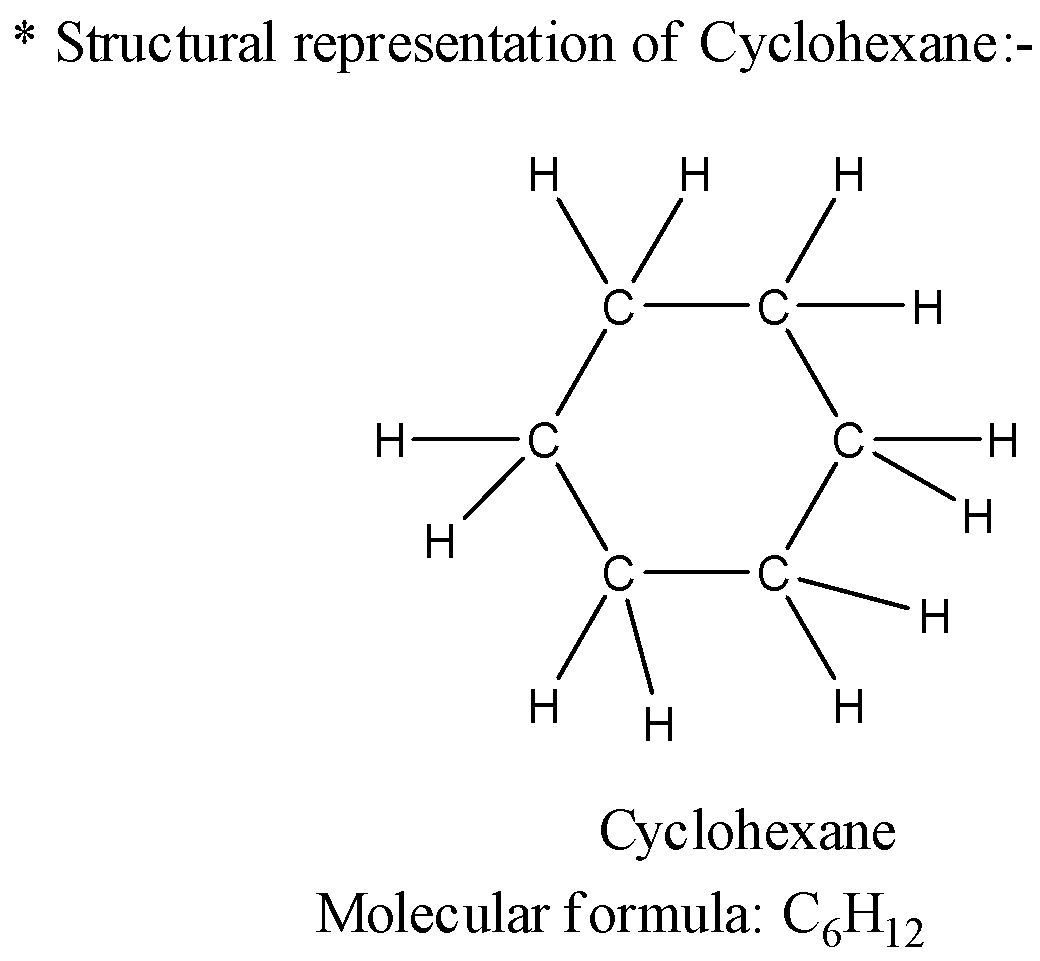
3) As we have analyzed the structures of both the compounds now let us see the difference between the structure of both compounds. The benzene has double bonds present in its structure which are conjugated to each other. This presence of the double bonds in conjugation makes the structure planar which means all the carbon atoms lie in the same plane.
4) The structure of cyclohexane has six carbon atoms that are bonded to each other by a single bond which makes the structure a cyclic structure. The structure of cyclohexane is not planar as a single bond has rotational properties and the structure can be present in more than one conformation.
Note:
The benzene is an aromatic structure due to the delocalization of pi bonds whereas cyclohexane is just a cyclic structure. The most stable conformation of the cyclohexane is the chair form. It may be present in boat form, half chair form, and twist chair form.
Complete step by step answer:
1) First of all, we will see the structures of benzene and cyclohexane and analyze their structures with respect to each other. The structure of benzene has molecular formula as ${C_6}{H_6}$ and the structure representation will be as below,

2) Now let us see the structure of the cyclohexane molecule which has molecular formula as ${C_6}{H_6}$ and the structure will be as below,

3) As we have analyzed the structures of both the compounds now let us see the difference between the structure of both compounds. The benzene has double bonds present in its structure which are conjugated to each other. This presence of the double bonds in conjugation makes the structure planar which means all the carbon atoms lie in the same plane.
4) The structure of cyclohexane has six carbon atoms that are bonded to each other by a single bond which makes the structure a cyclic structure. The structure of cyclohexane is not planar as a single bond has rotational properties and the structure can be present in more than one conformation.
Note:
The benzene is an aromatic structure due to the delocalization of pi bonds whereas cyclohexane is just a cyclic structure. The most stable conformation of the cyclohexane is the chair form. It may be present in boat form, half chair form, and twist chair form.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




