
Does the copper anode (increases /decreases/remains unchanged) in the weight?

Answer
538.8k+ views
Hint :Copper plate from the impure copper plate that is the anode of the electrode gets dissolved in the given copper sulphate solution .Copper ions making the anode plate a bit thinner and the copper ions present in the solution gets deposited at the cathode and hence make it thicker so the weight of copper anode decreases.
Complete Step By Step Answer:
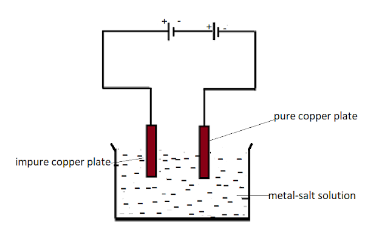
Here the given salt solution is copper sulphate salt solution. In the process of electrolysis when the connection is completed the salt solution starts breaking into copper and sulphate ions. The $ C{u^{2 + }} $ ions are the copper ions that go to the cathode and start depositing on it.
The electrode connected to the negative terminal is the cathode and the positive terminal electrode is the anode which is here impure copper plate. So the copper ions start dissolving in the solution from the anode and then due to positive charged particles they get attracted to the negative terminal that is cathode.
Slowly slowly all the $ C{u^{2 + }} $ ions gets deposited to the cathode and hence makes the anode thinner.
So the anode copper weight decreases by weight.
Note :
Copper ions make the anode plate a bit thinner and the copper ions present in the solution gets deposited at the cathode and hence make it thicker so the weight of copper anode decreases.
The salt solution should be the same metal salt solution whichever metal is used as electrode to complete the flow of current.
Complete Step By Step Answer:
Here the given salt solution is copper sulphate salt solution. In the process of electrolysis when the connection is completed the salt solution starts breaking into copper and sulphate ions. The $ C{u^{2 + }} $ ions are the copper ions that go to the cathode and start depositing on it.
The electrode connected to the negative terminal is the cathode and the positive terminal electrode is the anode which is here impure copper plate. So the copper ions start dissolving in the solution from the anode and then due to positive charged particles they get attracted to the negative terminal that is cathode.
Slowly slowly all the $ C{u^{2 + }} $ ions gets deposited to the cathode and hence makes the anode thinner.
So the anode copper weight decreases by weight.
Note :
Copper ions make the anode plate a bit thinner and the copper ions present in the solution gets deposited at the cathode and hence make it thicker so the weight of copper anode decreases.
The salt solution should be the same metal salt solution whichever metal is used as electrode to complete the flow of current.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




