
How does the atomic hydrogen or oxy-hydrogen torch function for cutting and welding purposes? Explain.
Answer
590.4k+ views
Hint: A very high temperature is required for welding and cutting of metals. Burning of hydrogen in air produces a very hot flame. Dissociation of dihydrogen into atomic hydrogen by electric arc followed by recombination of atoms releases a large amount of heat.
Complete answer:
Use of atomic hydrogen and oxy-hydrogen torch in welding and cutting purposes is explained below:
Oxy-hydrogen torch:
When hydrogen is burnt in oxygen, it burns with a very hot flame known as oxy-hydrogen flame. It has a temperature of about 2000-2500$^{o}C$.
The apparatus in which the flame is produced is known as oxy-hydrogen torch.
This temperature can easily melt platinum metal and therefore, it can be easily cut into pieces or welded together.
When oxy-hydrogen is directed on a stick of lime, the stick glows with a dazzling light, known as lime light.
Atomic hydrogen torch:
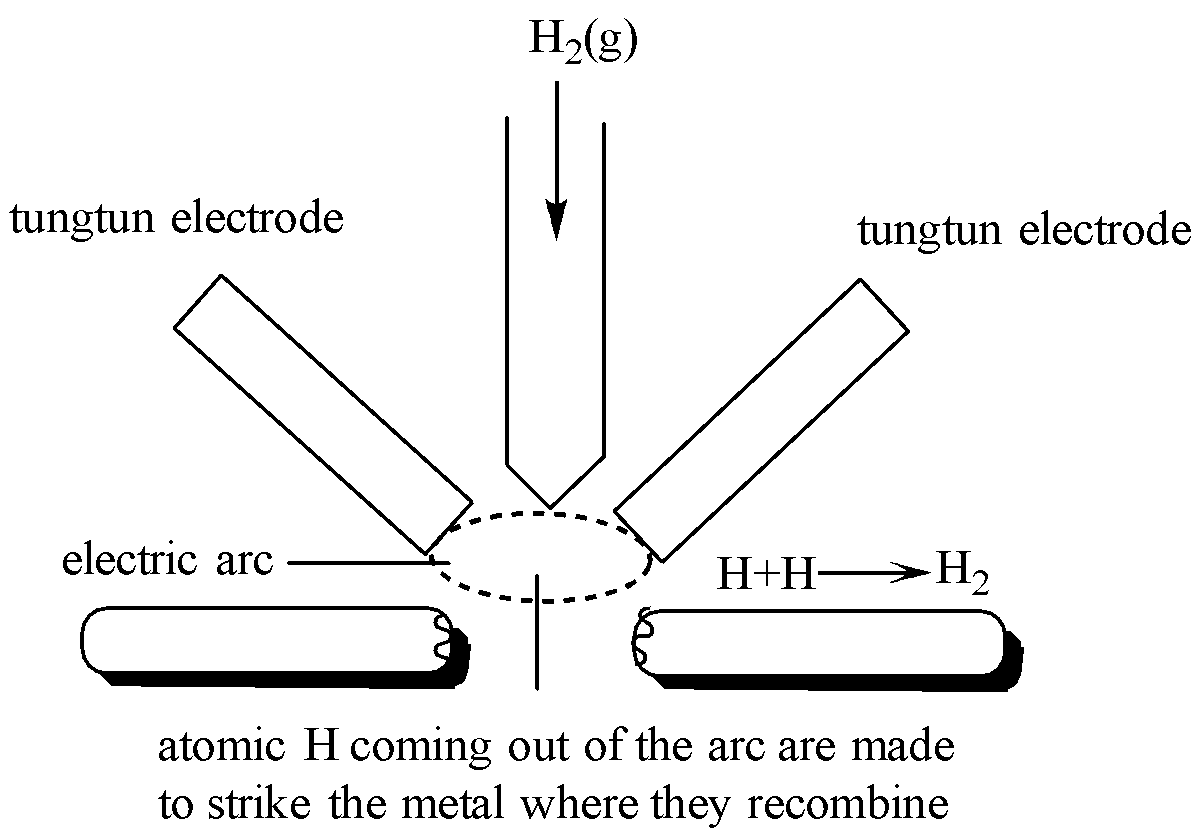
Irving Langmuir in 1927 devised an atomic hydrogen torch.
In this process, the molecular hydrogen is made to pass through an electric arc between tungsten electrodes where it gets heated to a very high temperature.
The electric arc breaks the molecular hydrogen into atomic hydrogens.
As soon as the atoms of hydrogen come out of the electric arc, they reunite to form molecular hydrogen. As result of this, a large of heat, i.e. 458.5 KJ/mol is liberated.
This amount of heat is enough to reach very high temperature in the range of 3500-4000$^{o}C$, which is suitable for cutting and welding purposes.
In welding, atomic hydrogens are made to recombine on the surface of the metal to be welded to form dihydrogen. This results in the generation of 3500-4000$^{o}C$ temperature.
Note:
It is to be noted here that oxy-hydrogen torch can only reach up to 2500$^{o}C$ temperature without the electric arc. Dissociation of dihydrogen to hydrogen atoms has enthalpy of dissociation, $\Delta {{H}_{D}}=485.1KJ/mol$. When these atomic hydrogens recombine on the metal surface, the same amount of energy is released as heat.
Complete answer:
Use of atomic hydrogen and oxy-hydrogen torch in welding and cutting purposes is explained below:
Oxy-hydrogen torch:
When hydrogen is burnt in oxygen, it burns with a very hot flame known as oxy-hydrogen flame. It has a temperature of about 2000-2500$^{o}C$.
The apparatus in which the flame is produced is known as oxy-hydrogen torch.
This temperature can easily melt platinum metal and therefore, it can be easily cut into pieces or welded together.
When oxy-hydrogen is directed on a stick of lime, the stick glows with a dazzling light, known as lime light.
Atomic hydrogen torch:
Irving Langmuir in 1927 devised an atomic hydrogen torch.
In this process, the molecular hydrogen is made to pass through an electric arc between tungsten electrodes where it gets heated to a very high temperature.
The electric arc breaks the molecular hydrogen into atomic hydrogens.
As soon as the atoms of hydrogen come out of the electric arc, they reunite to form molecular hydrogen. As result of this, a large of heat, i.e. 458.5 KJ/mol is liberated.
This amount of heat is enough to reach very high temperature in the range of 3500-4000$^{o}C$, which is suitable for cutting and welding purposes.
In welding, atomic hydrogens are made to recombine on the surface of the metal to be welded to form dihydrogen. This results in the generation of 3500-4000$^{o}C$ temperature.
- •
Note:
It is to be noted here that oxy-hydrogen torch can only reach up to 2500$^{o}C$ temperature without the electric arc. Dissociation of dihydrogen to hydrogen atoms has enthalpy of dissociation, $\Delta {{H}_{D}}=485.1KJ/mol$. When these atomic hydrogens recombine on the metal surface, the same amount of energy is released as heat.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




