
Does $ C{F_4} $ have a polar bond?
Answer
495k+ views
Hint: In this question, we should first find out the hybridization of a given compound by which we can find the shape of the compound. After that we will check whether the compound contains polar or nonpolar bonds. With the help of electronegativity of the elements in the compound, we can predict the polar or nonpolar nature of the bond.
Complete answer:
To find the polarity of the compound, there are following step needed:
Step-1: First of all we have to find the hybridization of in the compound which is:
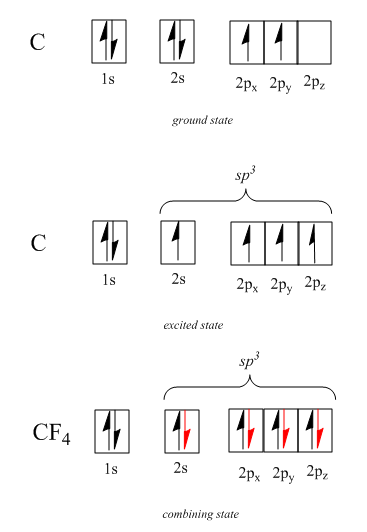
Hence the hybridization of carbon atom in $ C{F_4} $ compound is $ s{p^3} $ so the shape of compound $ C{F_4} $ is tetrahedral with four $ C - F $ bonds pointing towards the corners of a tetrahedron as shown in the following figure:
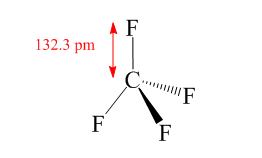
Step-2: Check whether each bond in the structure is polar or nonpolar which depends upon electronegativity difference. If electronegativity difference between the bonds is greater than $ 0.4 $ , then the bond will be polar but if it is less than $ 0.4 $ then it will be nonpolar.
As we know that Fluorine electronegativity is $ 4.0 $ whereas the electronegativity of Carbon is $ 2.5 $ , thus their electronegativity difference is more than $ 0.4 $ , i.e. 1.5 so each $ C - F $ bond is very polar. Hence, the bonds of the given compound are polar then the geometric sketch of the $ C{F_4} $ compound is:
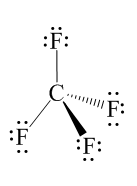
Note:
You can use Lewis structure as an alternative for prediction of polarity of the bond of the compound. Lewis structure is also known as Lewis dot formulas, are the diagrams which show the bonding between atoms of the molecule. It also shows the lone pairs of electrons that exist in the molecule.
Complete answer:
To find the polarity of the compound, there are following step needed:
Step-1: First of all we have to find the hybridization of in the compound which is:

Hence the hybridization of carbon atom in $ C{F_4} $ compound is $ s{p^3} $ so the shape of compound $ C{F_4} $ is tetrahedral with four $ C - F $ bonds pointing towards the corners of a tetrahedron as shown in the following figure:

Step-2: Check whether each bond in the structure is polar or nonpolar which depends upon electronegativity difference. If electronegativity difference between the bonds is greater than $ 0.4 $ , then the bond will be polar but if it is less than $ 0.4 $ then it will be nonpolar.
As we know that Fluorine electronegativity is $ 4.0 $ whereas the electronegativity of Carbon is $ 2.5 $ , thus their electronegativity difference is more than $ 0.4 $ , i.e. 1.5 so each $ C - F $ bond is very polar. Hence, the bonds of the given compound are polar then the geometric sketch of the $ C{F_4} $ compound is:

Note:
You can use Lewis structure as an alternative for prediction of polarity of the bond of the compound. Lewis structure is also known as Lewis dot formulas, are the diagrams which show the bonding between atoms of the molecule. It also shows the lone pairs of electrons that exist in the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




