
Distinguish between the meaning of the terms adsorption and absorption. Give one example of each.
Answer
524.3k+ views
Hint: During adsorption, the concentration of one substance on the surface of another substance is much higher than the concentration in the bulk. On the other hand, during absorption, there is uniform distribution of molecules of one substance throughout the body of another substance.
Complete step by step answer:
Major points of difference between adsorption and absorption are as follows –
Note:
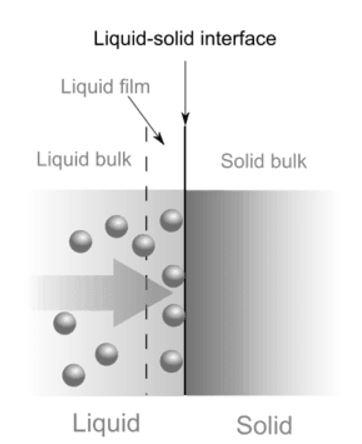
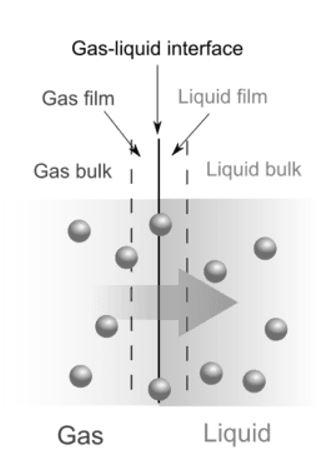
Adsorption is a surface phenomenon where molecules of adsorbate accumulate above the surface of the adsorbent. On the other hand, in absorption molecules of the ascorbate settle in between the molecules of the absorbent.
Complete step by step answer:
Major points of difference between adsorption and absorption are as follows –
| Adsorption | Absorption |
| A substance or energy is just attracted to the surface of another matter. | One substance is taken up into the physical structure of the other i.e., settles between the molecules of the other. |
| The material on whose surface these substances accumulate is called adsorbent and the substance that is adsorbed is called adsorbate. | The material into which molecules of the other one settle is called absorbent and the substance which settles is called absorbate. |
| Adsorption is a surface phenomenon. Concentration of the adsorbed substance is very high on the surface in comparison to bulk. | Concentration of absorbed substance is uniform throughout the absorbing material. Solid or liquid can absorb gaseous substances. |
| In case of chemisorption, specific reactions occur between the adsorbate and adsorbent. | No chemical reaction is occurring between the absorbate and the absorbent. |
| It is rapid in the beginning and slows down as it reaches near the equilibrium. | The entire process occurs at a uniform rate. |
| Examples: 1) Water vapours adsorbed by silica gel. 2) \[{\text{N}}{{\text{H}}_3}\]is adsorbed by charcoal. | Examples: 1) Water vapours absorbed by anhydrous \[{\text{CaC}}{{\text{l}}_2}\].2) \[{\text{N}}{{\text{H}}_3}\] is absorbed in water forming \[{\text{N}}{{\text{H}}_4}{\text{OH}}\]. |
| 
|
Note:
Adsorption is a surface phenomenon where molecules of adsorbate accumulate above the surface of the adsorbent. On the other hand, in absorption molecules of the ascorbate settle in between the molecules of the absorbent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




