
Discuss in brief about Phenolphthalein also do mention its uses and colour?
Answer
496.5k+ views
Hint: Phenolphthalein is a chemical compound with the formula ${C_{20}}{H_{14}}{O_4}$ and is often written as “phph” in shorthand notation. It is often used as an indicator in acid-base titration. It belongs to the class of dyes known as phthalein dyes.
Complete answer:
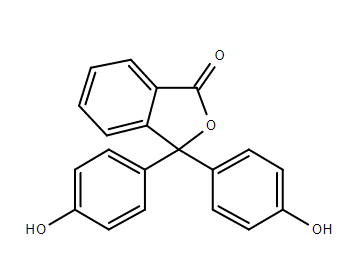
Phenolphthalein is slightly soluble in water and usually is dissolved in alcohols for use in experiments. Ionization occurs when a molecule gains or loses electrons, and this gives the molecule a negative or positive electric charge. Ionized molecules attract other molecules with the opposite charge and repel those with the same charge.
Phenolphthalein is a weak acid, which can lose ${H^ + }$ ions in solution. The nonionized phenolphthalein is colorless, the protonated phenolphthalein ion is orange and the deprotonated phenolphthalein ion is fuchsia.
Phenolphthalein common use is as an indicator in acid-base titrations. It also serves as a component of universal indicator, together with methyl red, bromothymol blue, and thymol blue.
Phenolphthalein adopts different states in aqueous solution as a result of pH changes.
Under strongly acidic conditions, it exists in protonated form $(php{h^ + })$, providing an orange coloration.
Between strongly acidic and slightly basic conditions, the lactone form $(phph)$ is colorless. The doubly deprotonated phenolate form gives the familiar pink color.
In strongly basic solutions, phenolphthalein is converted to its $phph{(OH)^{3 - }}$ form, and its pink color undergoes a rather slow fading reaction and becomes completely colorless above $13.0$pH.
The phenolphthalein indicator has two different structures based on whether it is in an alkali (pink) or acid (colorless) solution. Both structures absorb light in the ultraviolet region, a region not accessible for the human eye. However, the pink forms are also absorbed in the visible light spectrum.
The reason for the visible light absorption is the structure of the pink form of the phenolphthalein indicators. Due to ionization, the electrons in the molecule are more delocalized than in the colorless form. Briefly, delocalization is when electrons in a molecule are not associated with a single atom, and instead are spread over more than one atom.
Note:
Phenolphthalein is naturally colorless but turns pink in alkaline solution. The compounds remain colorless throughout the range of acidic pH levels but begin to turn pink at a pH level of \[8.2\]and continue to a bright magenta at pH \[10\] and above.
Complete answer:

Phenolphthalein is slightly soluble in water and usually is dissolved in alcohols for use in experiments. Ionization occurs when a molecule gains or loses electrons, and this gives the molecule a negative or positive electric charge. Ionized molecules attract other molecules with the opposite charge and repel those with the same charge.
Phenolphthalein is a weak acid, which can lose ${H^ + }$ ions in solution. The nonionized phenolphthalein is colorless, the protonated phenolphthalein ion is orange and the deprotonated phenolphthalein ion is fuchsia.
Phenolphthalein common use is as an indicator in acid-base titrations. It also serves as a component of universal indicator, together with methyl red, bromothymol blue, and thymol blue.
Phenolphthalein adopts different states in aqueous solution as a result of pH changes.
Under strongly acidic conditions, it exists in protonated form $(php{h^ + })$, providing an orange coloration.
Between strongly acidic and slightly basic conditions, the lactone form $(phph)$ is colorless. The doubly deprotonated phenolate form gives the familiar pink color.
In strongly basic solutions, phenolphthalein is converted to its $phph{(OH)^{3 - }}$ form, and its pink color undergoes a rather slow fading reaction and becomes completely colorless above $13.0$pH.
The phenolphthalein indicator has two different structures based on whether it is in an alkali (pink) or acid (colorless) solution. Both structures absorb light in the ultraviolet region, a region not accessible for the human eye. However, the pink forms are also absorbed in the visible light spectrum.
The reason for the visible light absorption is the structure of the pink form of the phenolphthalein indicators. Due to ionization, the electrons in the molecule are more delocalized than in the colorless form. Briefly, delocalization is when electrons in a molecule are not associated with a single atom, and instead are spread over more than one atom.
Note:
Phenolphthalein is naturally colorless but turns pink in alkaline solution. The compounds remain colorless throughout the range of acidic pH levels but begin to turn pink at a pH level of \[8.2\]and continue to a bright magenta at pH \[10\] and above.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a labelled diagram of the human heart and label class 11 biology CBSE

What is 1s 2s 2p 3s 3p class 11 chemistry CBSE




