
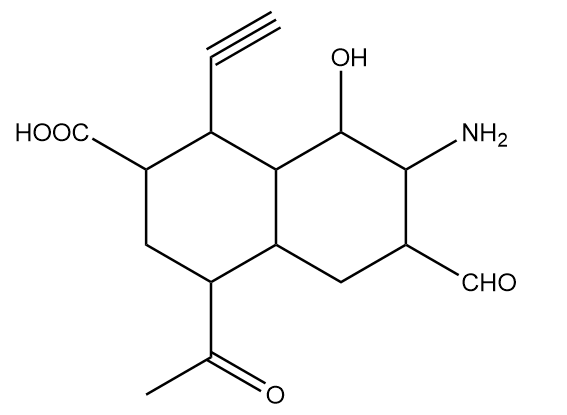
How many different functional groups are present in the given compound?
A. $6$
B. $5$
C. $4$
D. $3$

Answer
512.1k+ views
Hint: In organic chemistry, the functional groups are the substituent groups of atoms which are bonded to the specific molecules. These parts of a molecule are responsible to undergo distinctive chemical reactions and account for the different characteristic chemical properties for those molecules.
Complete answer:
In the given compound, the functional groups present are represented as follows:
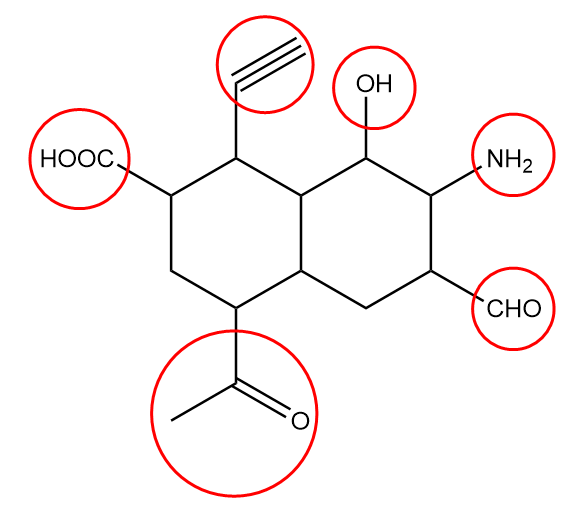
The name and general representation of the functional groups present are as follows:
1. Alcohol: It is generally represented as $R - OH$, where R represents the rest of the molecule and $OH$ represents a functional group.
2. Amine: It is generally represented as $R - N{H_2}$, where R represents the rest of the molecule and $N{H_2}$ represents a functional group.
3. Aldehyde: It is generally represented as $R - CHO$, where R represents the rest of the molecule and $CHO$ represents the aldehydic functional group.
4. Ketone: It is generally represented as follows:
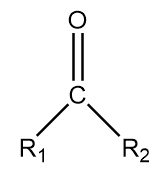
Where, ${R_1}$ and ${R_2}$ represent different alkyl groups and $ - C = O$ represents ketonic functional groups.
5. Carboxylic acid: It is generally represented as $R - COOH$, where R represents the alkyl group or the rest of the molecule and $COOH$ represents the functional group.
6. Alkyne: It is generally represented as ${R_1} - C \equiv C - {R_2}$, where ${R_1}$ and ${R_2}$ represents different alkyl groups and $C \equiv C$ represents the functional group.
Hence, the total number of functional groups present in the given compound $ = 6$. So, option (A) is the correct answer.
Note:
It is important to know that alkanes are not considered as functional groups, in fact an alkane is a compound or a molecule which lacks functional groups due to presence of carbon-carbon single bond which consist of only sigma character that is much stronger and stable than alkenes and alkynes.
Complete answer:
In the given compound, the functional groups present are represented as follows:

The name and general representation of the functional groups present are as follows:
1. Alcohol: It is generally represented as $R - OH$, where R represents the rest of the molecule and $OH$ represents a functional group.
2. Amine: It is generally represented as $R - N{H_2}$, where R represents the rest of the molecule and $N{H_2}$ represents a functional group.
3. Aldehyde: It is generally represented as $R - CHO$, where R represents the rest of the molecule and $CHO$ represents the aldehydic functional group.
4. Ketone: It is generally represented as follows:

Where, ${R_1}$ and ${R_2}$ represent different alkyl groups and $ - C = O$ represents ketonic functional groups.
5. Carboxylic acid: It is generally represented as $R - COOH$, where R represents the alkyl group or the rest of the molecule and $COOH$ represents the functional group.
6. Alkyne: It is generally represented as ${R_1} - C \equiv C - {R_2}$, where ${R_1}$ and ${R_2}$ represents different alkyl groups and $C \equiv C$ represents the functional group.
Hence, the total number of functional groups present in the given compound $ = 6$. So, option (A) is the correct answer.
Note:
It is important to know that alkanes are not considered as functional groups, in fact an alkane is a compound or a molecule which lacks functional groups due to presence of carbon-carbon single bond which consist of only sigma character that is much stronger and stable than alkenes and alkynes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




