
How did Iron come to earth (millions of years ago)?
Answer
566.1k+ views
Hint: Iron is originally made from fusion of elements in stars. Iron found on earth comes here and did so millions of years ago in the form of meteorites after a large stellar explosion of stars called supernova after the lifetime of a star is exhausted.
Complete answer:
Iron (Symbol: $_{26}^{56}Fe$, Electronic configuration: [ Ar ] $3{{d}^{6}}4{{s}^{2}}$) is a chemical element, which is the most abundant element on earth. Iron is made in the cores of stars due to fusion.
In the stars, when hydrogen present in the star's core is exhausted, the star starts its fusion chain. Hydrogen fuses with helium to form heavier elements, this process goes on and on, first if forms Carbon, then Neon, then Oxygen, then Silicone, at last it forms iron ash as an end product. The figure below shows the cross section of a star.
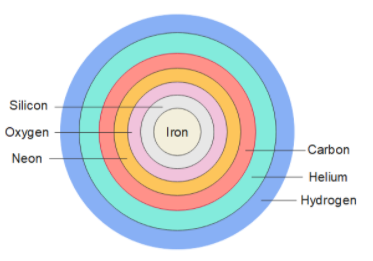
The fusion process releases energy. The elements heavier than iron and nickel are not formed because fusion requires energy.
The stars that have exhausted their fuel meaning when the lifetime of the star is over they explode in supernova explosion (powerful, luminous stellar explosion).
Due to the explosion, pieces of iron are blasted all around space. These fragments of iron come to earth in the form of meteors.
Earth’s core also contains iron from fusion of elements. Iron is one of the most abundant elements, by mass on earth. Iron is fourth most abundant in Earth’s crust. Pure iron is not found readily as iron oxidizes or rusts when comes in contact with moist air.
Note:
Iron is fourth most abundant in Earth’s crust, it comes after oxygen, silicon and aluminum. Iron exhibits ferromagnetism, i.e. it has high susceptibility to magnetization when magnetized by placing it in a magnetic field depending on the strength of the magnet.
Complete answer:
Iron (Symbol: $_{26}^{56}Fe$, Electronic configuration: [ Ar ] $3{{d}^{6}}4{{s}^{2}}$) is a chemical element, which is the most abundant element on earth. Iron is made in the cores of stars due to fusion.
In the stars, when hydrogen present in the star's core is exhausted, the star starts its fusion chain. Hydrogen fuses with helium to form heavier elements, this process goes on and on, first if forms Carbon, then Neon, then Oxygen, then Silicone, at last it forms iron ash as an end product. The figure below shows the cross section of a star.

The fusion process releases energy. The elements heavier than iron and nickel are not formed because fusion requires energy.
The stars that have exhausted their fuel meaning when the lifetime of the star is over they explode in supernova explosion (powerful, luminous stellar explosion).
Due to the explosion, pieces of iron are blasted all around space. These fragments of iron come to earth in the form of meteors.
Earth’s core also contains iron from fusion of elements. Iron is one of the most abundant elements, by mass on earth. Iron is fourth most abundant in Earth’s crust. Pure iron is not found readily as iron oxidizes or rusts when comes in contact with moist air.
Note:
Iron is fourth most abundant in Earth’s crust, it comes after oxygen, silicon and aluminum. Iron exhibits ferromagnetism, i.e. it has high susceptibility to magnetization when magnetized by placing it in a magnetic field depending on the strength of the magnet.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE

Explain sex determination in humans with line diag class 12 biology CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE

What is myopia and hypermetropia How are they corrected class 12 physics CBSE




