
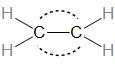
Diagram for bonding in ethene with ${\text{s}}{{\text{p}}^2}$ hybridisation.
Answer
578.1k+ views
Hint: To answer this question you must recall the VSEPR (Valence shell electron pair repulsion) theory. It suggests that all valence shell electron pairs surrounding the central atom arrange themselves in such a manner so as to be as far away from each other as possible.
Complete step by step answer:
An Ethene molecule has a double bond between the two carbon atoms and single bonds between the carbon and hydrogen atoms. The aim for each carbon atom is to complete an octet and for the hydrogen to complete a duplet. Thus each hydrogen forms one bond with a carbon atom and each carbon forms two single bonds with hydrogen atoms and a double bond with the other carbon.
We know that in ethene, out of four valence electrons of carbon, one is used for double bond formation. So the hybridisation will be ${\text{s}}{{\text{p}}^2}$ and there will be one unpaired electron in the unhybridized ${\text{p}}$-orbital.
The VSEPR Theory is used to predict the bond angles and spatial positions of the carbon and hydrogen atoms. Each carbon forms three single bonds and thus the bond angle is ${120^{\text{o}}}$. As a result, ethene is a planar molecule with the overlapping ${\text{p}}$ orbitals perpendicular to the plane of the carbon carbon single bond.
We can draw the diagram of the ethene molecule as:
Note:
During bond formation, the atomic orbitals of an atom are mixed in such a manner as to produce equivalent orbitals. This mixing of orbitals is known as hybridisation. The arrangement of these hybrid orbitals according to the VSEPR theory gives us the shape of the molecule
Complete step by step answer:
An Ethene molecule has a double bond between the two carbon atoms and single bonds between the carbon and hydrogen atoms. The aim for each carbon atom is to complete an octet and for the hydrogen to complete a duplet. Thus each hydrogen forms one bond with a carbon atom and each carbon forms two single bonds with hydrogen atoms and a double bond with the other carbon.
We know that in ethene, out of four valence electrons of carbon, one is used for double bond formation. So the hybridisation will be ${\text{s}}{{\text{p}}^2}$ and there will be one unpaired electron in the unhybridized ${\text{p}}$-orbital.
The VSEPR Theory is used to predict the bond angles and spatial positions of the carbon and hydrogen atoms. Each carbon forms three single bonds and thus the bond angle is ${120^{\text{o}}}$. As a result, ethene is a planar molecule with the overlapping ${\text{p}}$ orbitals perpendicular to the plane of the carbon carbon single bond.
We can draw the diagram of the ethene molecule as:

Note:
During bond formation, the atomic orbitals of an atom are mixed in such a manner as to produce equivalent orbitals. This mixing of orbitals is known as hybridisation. The arrangement of these hybrid orbitals according to the VSEPR theory gives us the shape of the molecule
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




