
Determine the bond order of the $C - O$ bond in the following molecules, and then arrange the $C - O$ bond lengths in increasing order? $CO$, $C{O^ + }$ ,$C{O^{2 + }}$ ,$C{O_2}$ ,$C{O_3}^{2 - }$.
Answer
495.9k+ views
Hint: For this question we should know about bond order, bond length and relation between them. As we know that the shorter the bond length, the stronger will be the bond and the stronger the bond, higher will be the bond order. So this implies that shorter the bond length, higher will be the bond order.
Formula Used:
Bond order (BO) = $\dfrac{1}{2}(Bonding\;electrons - Antibonding\,electrons)$
Complete answer:
Bond Order of the compound is the half of the difference between the number of bonding and antibonding electrons as shown in the formula. So with the help of Molecular Orbital Diagram, we can find out the total number of bonding and antibonding electrons present in the given compounds, i.e. $CO$, $C{O^ + }$ ,$C{O^{2 + }}$ ,$C{O_2}$ ,$C{O_3}^{2 - }$ .
Molecular Orbital Diagram of $CO$ :
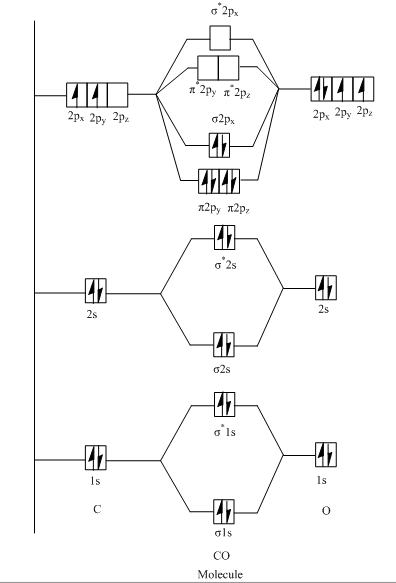
From above diagram, it is clear that number of bonding electrons is six (present two electrons in $2\sigma $ , 4 in $1\pi $ and 2 in $3\sigma $ ) whereas number of antibonding electrons is two (present in $2{\sigma ^*}$ ).
So the bond orders of $CO$ is:
BO= $\dfrac{1}{2}(bonding\;electrons - antibonding\;electrons)$
$ \Rightarrow \dfrac{1}{2}([2 + 2 \times 2 + 2] - [2])$
$ \Rightarrow 3$
Now, for $C{O^ + }$ Cation, we know that one electron is removed from a bonding MO. Then the bond order will decrease. So by using the same formula we get a bond order of $C{O^ + }$ is $2.5$ .
For $C{O^{2 + }}$ Dication, it has one more electron removed from $C{O^ + }$ , so the bond order is $2$ .
For $C{O_2}$ molecule, the structure of $C{O_2}$ :
So, it has double bonds and hence has a bond order of $2$ .
For carbonate anions, we should consider resonance delocalization. The structure of $C{O_3}^{2 - }$ :
Here, there will be $2\pi $ electrons delocalized onto three bonds, by making an average of $\dfrac{2}{3}$ of a $\pi $ electron, giving a $\pi $ bond order of $\dfrac{1}{3}$ . Where $\sigma $ bonds have a bond order of $1$ , the total bond order of carbonate anion is $1.\overline {33} $ .
Since the higher the bond order, the shorter the bond length will be, so the increasing order of the given compounds according to their bond length is:
${r_{CO}} < {r_{C{O^ + }}} < [{r_{C{O^{2 + }}}} = {r_{C{O_2}}}] < {r_{C{O_3}^{2 - }}}$
Note:
Bond order was invented by Linus Pauling, and is defined as the difference between the number of bonds and antibonds. The bond number itself is the number of electron pairs between a pair of atoms.
Formula Used:
Bond order (BO) = $\dfrac{1}{2}(Bonding\;electrons - Antibonding\,electrons)$
Complete answer:
Bond Order of the compound is the half of the difference between the number of bonding and antibonding electrons as shown in the formula. So with the help of Molecular Orbital Diagram, we can find out the total number of bonding and antibonding electrons present in the given compounds, i.e. $CO$, $C{O^ + }$ ,$C{O^{2 + }}$ ,$C{O_2}$ ,$C{O_3}^{2 - }$ .
Molecular Orbital Diagram of $CO$ :

From above diagram, it is clear that number of bonding electrons is six (present two electrons in $2\sigma $ , 4 in $1\pi $ and 2 in $3\sigma $ ) whereas number of antibonding electrons is two (present in $2{\sigma ^*}$ ).
So the bond orders of $CO$ is:
BO= $\dfrac{1}{2}(bonding\;electrons - antibonding\;electrons)$
$ \Rightarrow \dfrac{1}{2}([2 + 2 \times 2 + 2] - [2])$
$ \Rightarrow 3$
Now, for $C{O^ + }$ Cation, we know that one electron is removed from a bonding MO. Then the bond order will decrease. So by using the same formula we get a bond order of $C{O^ + }$ is $2.5$ .
For $C{O^{2 + }}$ Dication, it has one more electron removed from $C{O^ + }$ , so the bond order is $2$ .
For $C{O_2}$ molecule, the structure of $C{O_2}$ :
So, it has double bonds and hence has a bond order of $2$ .
For carbonate anions, we should consider resonance delocalization. The structure of $C{O_3}^{2 - }$ :
Here, there will be $2\pi $ electrons delocalized onto three bonds, by making an average of $\dfrac{2}{3}$ of a $\pi $ electron, giving a $\pi $ bond order of $\dfrac{1}{3}$ . Where $\sigma $ bonds have a bond order of $1$ , the total bond order of carbonate anion is $1.\overline {33} $ .
Since the higher the bond order, the shorter the bond length will be, so the increasing order of the given compounds according to their bond length is:
${r_{CO}} < {r_{C{O^ + }}} < [{r_{C{O^{2 + }}}} = {r_{C{O_2}}}] < {r_{C{O_3}^{2 - }}}$
Note:
Bond order was invented by Linus Pauling, and is defined as the difference between the number of bonds and antibonds. The bond number itself is the number of electron pairs between a pair of atoms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




