
How do you determine the acidity of aromatic compounds?
Answer
556.8k+ views
Hint:Acidity is defined as the tendency of a compound to release the Hydronium ion $\left( {{H}^{+}} \right)$ easily. We know that aromatic compounds are those which follow Huckel’s rule. Aromaticity plays a big role in determining the acidity of aromatic compounds.
Complete answer:
Aromatic compounds are those which fulfils the huckel’s rule. As per huckel an aromatic compound is one which contains $\left( 4n+2 \right)\pi $ electrons in the ring. Where n= any whole number. Along with it, aromatic compounds should be planar and should show complete dissociation of $\pi $ electrons.
We can determine the acidity of any aromatic compound by measuring its $Ka$. Alternatively, $Ka$ may be also expressed as $pKa$ which is defined as $pKa=-\log Ka$. Smaller the $pKa$ value, stronger is the acid.
But we should remember that acidity or $Ka$ values depend on various factors. Few are as follows.
1. Resonance: we know that in resonance the delocalization of the pie electrons takes place and as a result due to this the stability of the aromatic compound increases.
For example: Phenol is a resonance hybrid of following structures.
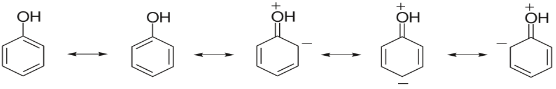
It is clear that three structures have positive charge on oxygen of the -OH group. This oxygen attracts the electron pair of OH towards itself, making it easier for the hydrogen to escape. And we know that if hydrogen can be easily escaped then the compound is more acidic.
Also, the phenoxide ion formed after the release of hydrogen is much more stable by resonance itself. And we know if the product formed is more stable then the reaction is favored. So, we can conclude that Phenol will be more acidic as the phenoxide ion formed after release of hydrogen is stabilized by resonance.
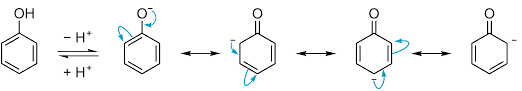
It should be noted that phenoxide ion is more stable than phenol as there is no charge separation in it, unlike phenol.
2. Electron withdrawing group: Such groups withdraw electrons from the aromatic ring, disperses the charge and as result makes the compound more acidic and thus increasing the value of $Ka$. Examples of such groups are $N{{O}_{2}},\,CN,\,etc$
3. Electron releasing group: Such groups, donates the electron in the ring thus intensifying the negative charge and as a result decreases the acidity of the compound. Examples of such groups are $-N{{H}_{2}},\,-OR,\,-R$
Note: It should be noted that while determining the acidity of the compound all the above factors should be kept in mind. Also, if more than one electron withdrawing or donating group is attached then always consider the more stronger group.
Complete answer:
Aromatic compounds are those which fulfils the huckel’s rule. As per huckel an aromatic compound is one which contains $\left( 4n+2 \right)\pi $ electrons in the ring. Where n= any whole number. Along with it, aromatic compounds should be planar and should show complete dissociation of $\pi $ electrons.
We can determine the acidity of any aromatic compound by measuring its $Ka$. Alternatively, $Ka$ may be also expressed as $pKa$ which is defined as $pKa=-\log Ka$. Smaller the $pKa$ value, stronger is the acid.
But we should remember that acidity or $Ka$ values depend on various factors. Few are as follows.
1. Resonance: we know that in resonance the delocalization of the pie electrons takes place and as a result due to this the stability of the aromatic compound increases.
For example: Phenol is a resonance hybrid of following structures.

It is clear that three structures have positive charge on oxygen of the -OH group. This oxygen attracts the electron pair of OH towards itself, making it easier for the hydrogen to escape. And we know that if hydrogen can be easily escaped then the compound is more acidic.
Also, the phenoxide ion formed after the release of hydrogen is much more stable by resonance itself. And we know if the product formed is more stable then the reaction is favored. So, we can conclude that Phenol will be more acidic as the phenoxide ion formed after release of hydrogen is stabilized by resonance.

It should be noted that phenoxide ion is more stable than phenol as there is no charge separation in it, unlike phenol.
2. Electron withdrawing group: Such groups withdraw electrons from the aromatic ring, disperses the charge and as result makes the compound more acidic and thus increasing the value of $Ka$. Examples of such groups are $N{{O}_{2}},\,CN,\,etc$
3. Electron releasing group: Such groups, donates the electron in the ring thus intensifying the negative charge and as a result decreases the acidity of the compound. Examples of such groups are $-N{{H}_{2}},\,-OR,\,-R$
Note: It should be noted that while determining the acidity of the compound all the above factors should be kept in mind. Also, if more than one electron withdrawing or donating group is attached then always consider the more stronger group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




