
Describe a method for the identification of primary, secondary and tertiary amines. Also write chemical equations of the reactions involved.
Answer
600k+ views
Hint: To answer this question we have to recall the difference between the three amines. We should also remember the Hinsberg test which differentiates between the three.
Complete step by step solution:
Aliphatic amines occur in nature, principally as products of the protein material, but they are also present in living tissue. Amines are classified as primary, secondary, or tertiary according to the number of carbons bonded directly to the nitrogen atom. Primary amines have one carbon bonded to the nitrogen. Secondary amines have two carbons bonded to the nitrogen, and tertiary amines have three carbons bonded to the nitrogen
Primary, secondary and tertiary amines react differently with Hinsberg reagent (Benzosulphonyl chloride) in presence of an aqueous alkali. Hence, we generally use the Hinsberg test to distinguish between amines.
* Primary amine reacts with Hinsberg reagent to form a N-alkylbenzenesulphonyl amide which is observed to be soluble in the aqueous alkali.
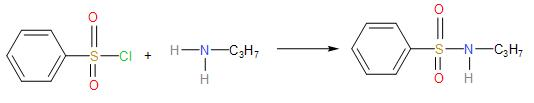
Since, there is a strong electron withdrawing group in the sulphonamide; the hydrogen atom attached to the nitrogen is easily released as proton. Hence, it dissolves the alkali.
* But, we see that secondary amines react with Hinsberg reagent to produce a sulphonamide which remains insoluble in the alkali.
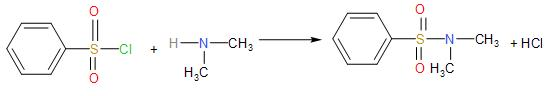
Since, there is no hydrogen atom attached to the nitrogen atom of sulphonamide, it does not dissolve in the alkali.
* In contrast to these Tertiary amines do not react with Hinsberg reagent at all.
Note: We can use another method to distinguish between amines. This is known as the Hoffman method. Primary amine yields a solid product with ethyl oxalate, secondary amine yields a liquid product whereas no reaction takes place in case of tertiary amines.
Complete step by step solution:
Aliphatic amines occur in nature, principally as products of the protein material, but they are also present in living tissue. Amines are classified as primary, secondary, or tertiary according to the number of carbons bonded directly to the nitrogen atom. Primary amines have one carbon bonded to the nitrogen. Secondary amines have two carbons bonded to the nitrogen, and tertiary amines have three carbons bonded to the nitrogen
Primary, secondary and tertiary amines react differently with Hinsberg reagent (Benzosulphonyl chloride) in presence of an aqueous alkali. Hence, we generally use the Hinsberg test to distinguish between amines.
* Primary amine reacts with Hinsberg reagent to form a N-alkylbenzenesulphonyl amide which is observed to be soluble in the aqueous alkali.
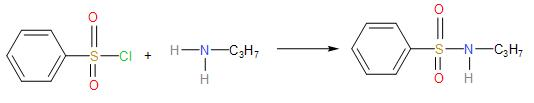
Since, there is a strong electron withdrawing group in the sulphonamide; the hydrogen atom attached to the nitrogen is easily released as proton. Hence, it dissolves the alkali.
* But, we see that secondary amines react with Hinsberg reagent to produce a sulphonamide which remains insoluble in the alkali.

Since, there is no hydrogen atom attached to the nitrogen atom of sulphonamide, it does not dissolve in the alkali.
* In contrast to these Tertiary amines do not react with Hinsberg reagent at all.
Note: We can use another method to distinguish between amines. This is known as the Hoffman method. Primary amine yields a solid product with ethyl oxalate, secondary amine yields a liquid product whereas no reaction takes place in case of tertiary amines.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




