
Define the following term:
Zeta potential.
Answer
584.7k+ views
Hint: Zeta potential is also known as Electrokinetic. It is used to define or explain the process of preferential adsorption of ions from solution in the electrical charge on colloidal particles. There are two layers: the fixed layer and the diffused layer.
Complete step by step answer:
The stability of the colloidal sol is defined by some properties. The electrical charge on the colloidal particle is one of them. The electrical charge on the colloidal particle is explained by the process of preferential adsorption of ions from the solution. During the preparation of colloidal sol, an ionic colloidal adsorb ions common to its lattice.
It can be explained by the colloidal sol $AgI$ is prepared by adding $KI$ solution to the $AgN{{O}_{3}}$ solution till $KI$ is in slight excess, iodide ion (${{I}^{-}}$) will be absorbed in the surface of $AgI$ particles thereby giving them a negative charge:
$AgI+{{I}^{-}}\to AgI:{{I}^{-}}$
The same can be done if the colloidal sol $AgI$ is prepared by adding $AgN{{O}_{3}}$ solution to the $KI$ solution till $AgN{{O}_{3}}$ is in slight excess, iodide ion ($A{{g}^{+}}$ ) will be absorbed in the surface of $AgI$ particles thereby giving them a positive charge:
$AgI+A{{g}^{+}}\to AgI:A{{g}^{+}}$
This can be explained when one type of ions of the electrolyte is adsorbed on the surface of the colloidal particles, there is a formation of a 'fixed layer'. This layer attracts the counter ions from the medium forming a mobile layer known as 'diffused layer'. This potential difference is known as electrokinetic or zeta potential.
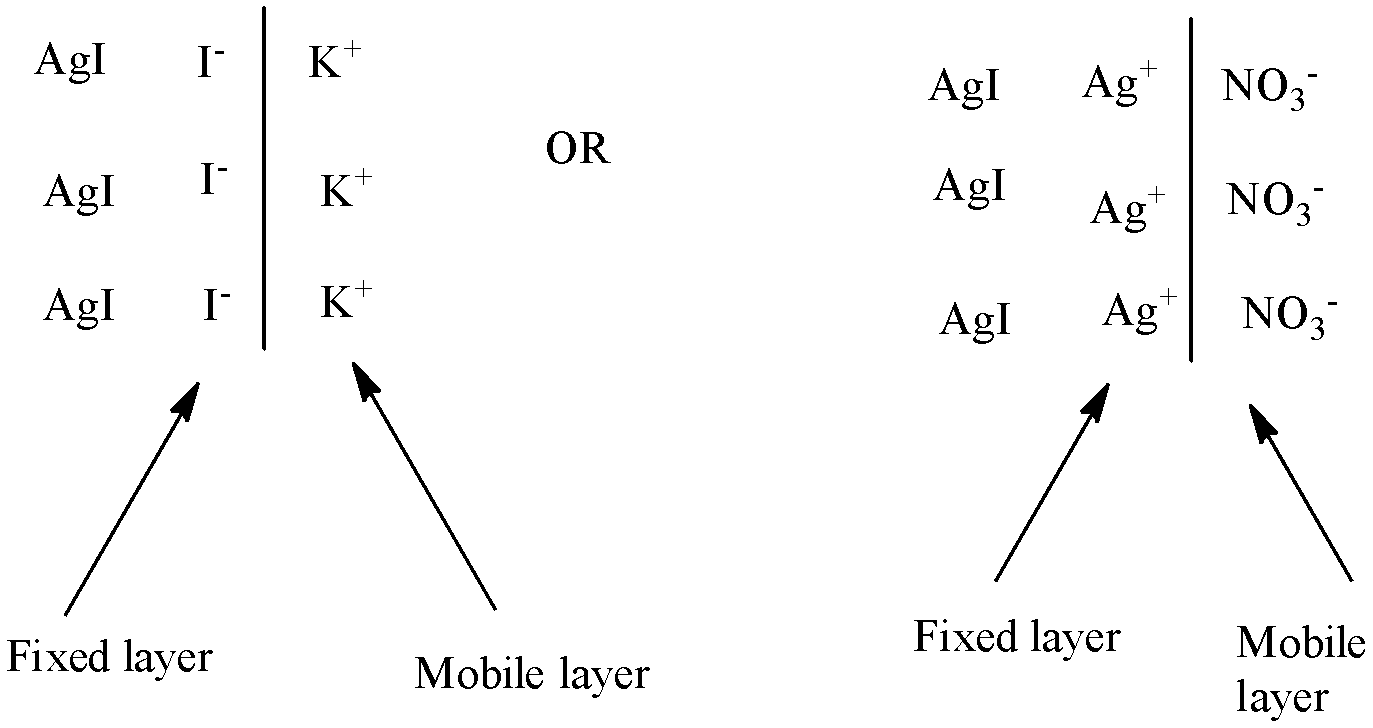
Note: Helmholtz electrical double layer is the double layer of opposite charge formed in the zeta potential layer. So, the left-out excess ions remain in the solution thereby giving equal and opposite charge to the dispersion medium.
Complete step by step answer:
The stability of the colloidal sol is defined by some properties. The electrical charge on the colloidal particle is one of them. The electrical charge on the colloidal particle is explained by the process of preferential adsorption of ions from the solution. During the preparation of colloidal sol, an ionic colloidal adsorb ions common to its lattice.
It can be explained by the colloidal sol $AgI$ is prepared by adding $KI$ solution to the $AgN{{O}_{3}}$ solution till $KI$ is in slight excess, iodide ion (${{I}^{-}}$) will be absorbed in the surface of $AgI$ particles thereby giving them a negative charge:
$AgI+{{I}^{-}}\to AgI:{{I}^{-}}$
The same can be done if the colloidal sol $AgI$ is prepared by adding $AgN{{O}_{3}}$ solution to the $KI$ solution till $AgN{{O}_{3}}$ is in slight excess, iodide ion ($A{{g}^{+}}$ ) will be absorbed in the surface of $AgI$ particles thereby giving them a positive charge:
$AgI+A{{g}^{+}}\to AgI:A{{g}^{+}}$
This can be explained when one type of ions of the electrolyte is adsorbed on the surface of the colloidal particles, there is a formation of a 'fixed layer'. This layer attracts the counter ions from the medium forming a mobile layer known as 'diffused layer'. This potential difference is known as electrokinetic or zeta potential.

Note: Helmholtz electrical double layer is the double layer of opposite charge formed in the zeta potential layer. So, the left-out excess ions remain in the solution thereby giving equal and opposite charge to the dispersion medium.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




