
Define carbohydrates. What are reducing and non-reducing sugars?
Answer
596.1k+ views
Hint: Maltose and lactose are reducing sugars, whereas sucrose is an example of a non-reducing sugar. They describe the ability to get reduced by some reducing agents.
Complete step by step solution:
Let us first begin with the definition of carbohydrates before moving on to the distinction between reducing and non-reducing sugars.
- A carbohydrate is a biomolecule which consists of carbon, hydrogen and oxygen atoms, usually with a hydrogen–oxygen atom ratio of 2:1 and thus it can be given the empirical formula \[{{C}_{m}}{{({{H}_{2}}O)}_{n}}~\] (where m may be different from n). This formula holds true for monosaccharides. However some exceptions are there; for example, Deoxyribose which is a sugar component of DNA, has the empirical formula given by \[{{C}_{5}}{{H}_{10}}{{O}_{4}}\]. The carbohydrates though are technically hydrated forms of carbon but structurally it is more accurate to view them as aldoses and ketoses.
- This term is most common in biochemistry where it is a synonym of saccharide.
- Now let us look at reducing and non-reducing sugars in detail.
- Reducing sugars are types of carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups in their structures. All monosaccharides and some disaccharides are reducing sugars.
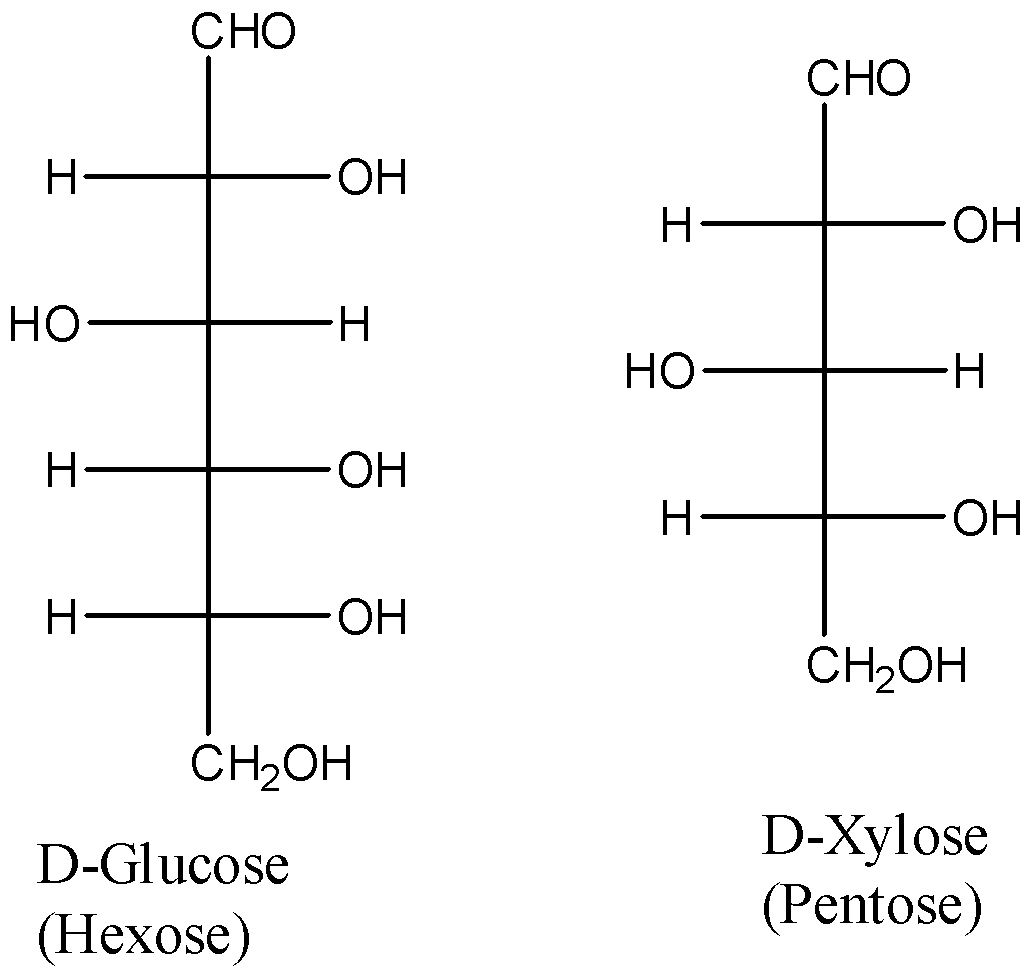
- Reducing sugars can be oxidized by those compounds that are weak oxidizing agents. In aqueous medium, reducing sugars give one or more compounds containing an aldehyde group. This is a characteristic property of reducing sugars.
- Nonreducing sugars are carbohydrate compounds that cannot act as reducing agents due to the absence of free aldehyde groups or free ketone groups in their structure. Some disaccharides and all polysaccharides are reducing sugars. In basic aqueous media, nonreducing sugars are not able to generate any compounds containing an aldehyde group.
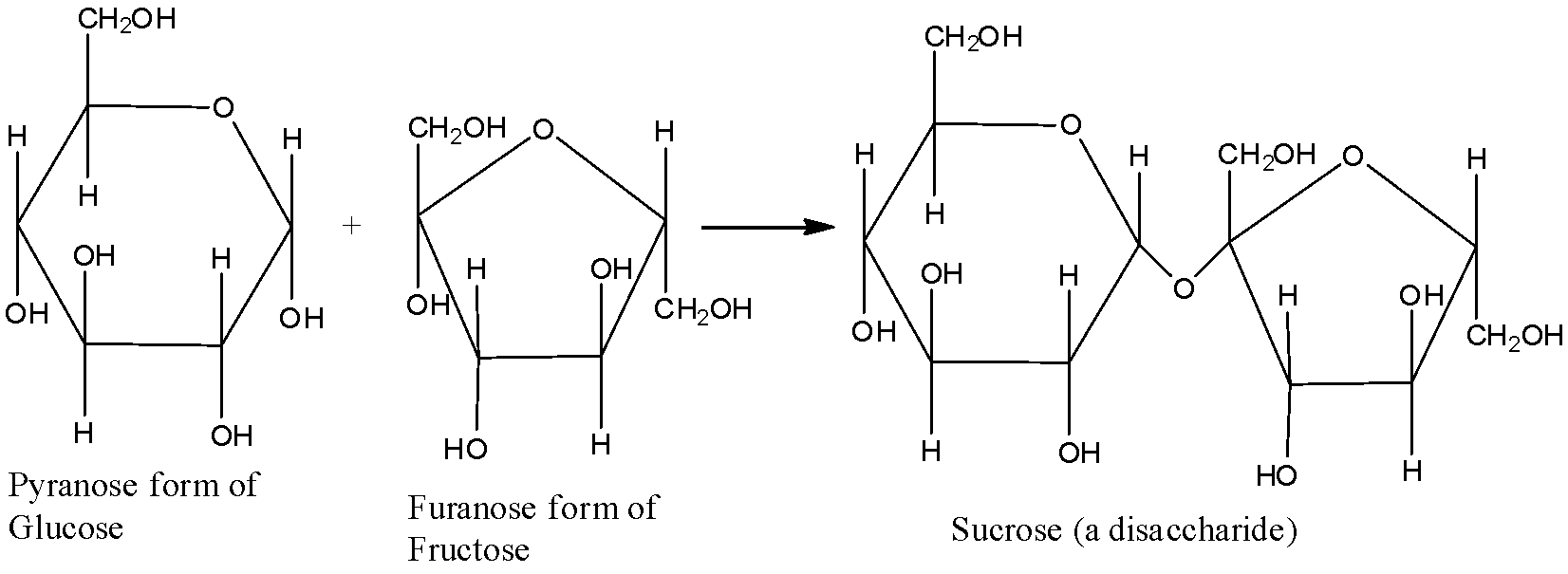
Note: The major difference between non-reducing and reducing sugar is that non-reducing sugars do not have free aldehyde or ketone groups whereas reducing sugars have free aldehyde or ketone groups. This is an extremely important distinction and must be always remembered.
Complete step by step solution:
Let us first begin with the definition of carbohydrates before moving on to the distinction between reducing and non-reducing sugars.
- A carbohydrate is a biomolecule which consists of carbon, hydrogen and oxygen atoms, usually with a hydrogen–oxygen atom ratio of 2:1 and thus it can be given the empirical formula \[{{C}_{m}}{{({{H}_{2}}O)}_{n}}~\] (where m may be different from n). This formula holds true for monosaccharides. However some exceptions are there; for example, Deoxyribose which is a sugar component of DNA, has the empirical formula given by \[{{C}_{5}}{{H}_{10}}{{O}_{4}}\]. The carbohydrates though are technically hydrated forms of carbon but structurally it is more accurate to view them as aldoses and ketoses.
- This term is most common in biochemistry where it is a synonym of saccharide.
- Now let us look at reducing and non-reducing sugars in detail.
- Reducing sugars are types of carbohydrates that can act as reducing agents due to the presence of free aldehyde groups or free ketone groups in their structures. All monosaccharides and some disaccharides are reducing sugars.

- Reducing sugars can be oxidized by those compounds that are weak oxidizing agents. In aqueous medium, reducing sugars give one or more compounds containing an aldehyde group. This is a characteristic property of reducing sugars.
- Nonreducing sugars are carbohydrate compounds that cannot act as reducing agents due to the absence of free aldehyde groups or free ketone groups in their structure. Some disaccharides and all polysaccharides are reducing sugars. In basic aqueous media, nonreducing sugars are not able to generate any compounds containing an aldehyde group.

Note: The major difference between non-reducing and reducing sugar is that non-reducing sugars do not have free aldehyde or ketone groups whereas reducing sugars have free aldehyde or ketone groups. This is an extremely important distinction and must be always remembered.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




