
Dacron is an example of a/an:
(A)- elastomer
(B)- fibre
(C)- thermoplastic
(D)- thermosetting polymer
Answer
576k+ views
Hint: The type of polymer is determined by the type of repeating unit found in the Dacron polymer and its formation, which will describe its properties of strength and stiffness. Thus, we can wisely choose the right option.
Complete step by step answer:
Dacron is a polymer which is formed by the condensation polymerisation of the two monomers, that is ethylene glycol and terephthalic acid. As the two monomers are diol and dicarboxylic acid respectively.
They combine together on heating in presence of the catalyst (zinc acetate antimony trioxide) leading to the loss of water molecule to form the repeating unit, ethylene terephthalate, which is an ester. Thus, the process is known as esterification.
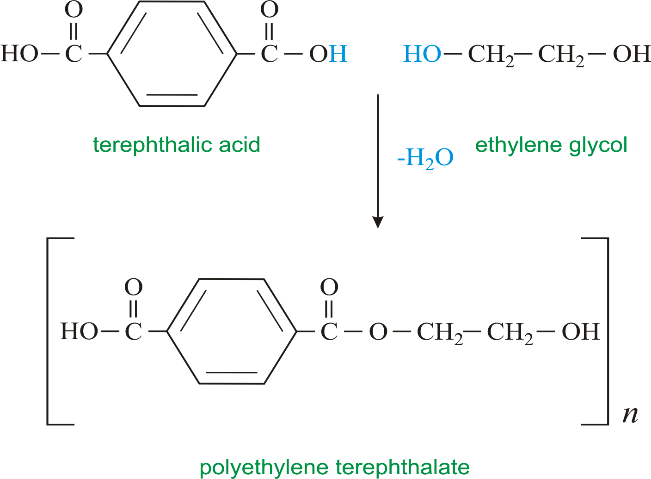
This, polyethylene terephthalate or terylene, which is a polyester. It can be used in both the making of clothes and plastic bottles. As the molten form can be directly made into fibres which are spun into yarns. Also, it can be solidified and moulded into plastic bottles.
This is due to the stiffness and the strength found in the polyester in presence of the aromatic rings in it, which makes it useful in the textile industry to produce high-quality materials.
As it is crease-resistance, durable and provides thermal comfort through its moisture-wicking property. It is also blended with other fibres, wherein it reinforces and enhances their property.
So, the correct answer is “Option B”.
Note: The Dacron is thus used for manufacturing a variety of things like the garments which we wear, the furniture supplies (like pillow cases) and also in marine application as a base material for various purposes (like sail cloth, coveralls) etc.
Complete step by step answer:
Dacron is a polymer which is formed by the condensation polymerisation of the two monomers, that is ethylene glycol and terephthalic acid. As the two monomers are diol and dicarboxylic acid respectively.
They combine together on heating in presence of the catalyst (zinc acetate antimony trioxide) leading to the loss of water molecule to form the repeating unit, ethylene terephthalate, which is an ester. Thus, the process is known as esterification.

This, polyethylene terephthalate or terylene, which is a polyester. It can be used in both the making of clothes and plastic bottles. As the molten form can be directly made into fibres which are spun into yarns. Also, it can be solidified and moulded into plastic bottles.
This is due to the stiffness and the strength found in the polyester in presence of the aromatic rings in it, which makes it useful in the textile industry to produce high-quality materials.
As it is crease-resistance, durable and provides thermal comfort through its moisture-wicking property. It is also blended with other fibres, wherein it reinforces and enhances their property.
So, the correct answer is “Option B”.
Note: The Dacron is thus used for manufacturing a variety of things like the garments which we wear, the furniture supplies (like pillow cases) and also in marine application as a base material for various purposes (like sail cloth, coveralls) etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




