
How many cyclopentane structures (excluding stereoisomers) are possible for${{C}_{7}}{{H}_{14}}$ .
Answer
542.1k+ views
Hint:Cyclopentane is an organic chemical containing five carbon atoms present in a cyclic ring. Generally cyclic organic compounds up to five membered rings are less stable in nature when compared to aliphatic hydrocarbons of the same length.
Complete step-by-step answer:- In the question it is asked to draw the structures of cyclopentane that are possible with the molecular formula of ${{C}_{7}}{{H}_{14}}$ by excluding the stereoisomers.
- In the given molecular formula there are seven carbon atoms and fourteen hydrogen atoms are present.
- The basic structure of cyclopentane is as follows.
- Five carbon atoms are going to attached end to end each other.
- Stereoisomers are the isomers that have the same molecular formula and same sequence of bonds but they differ in three dimensional orientations.
- The possible cyclopentane structures with the molecular formula are as follows.
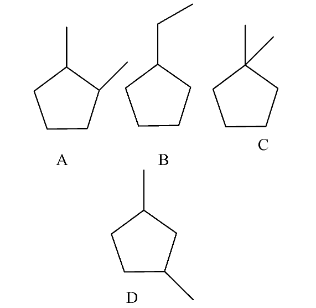
- There are only four possible cyclopentane structures by excluding the stereoisomers with the molecular formula of ${{C}_{7}}{{H}_{14}}$.
- Structure D and B can form two stereoisomers.
- Structure C and A can form one more stereoisomer with the same molecular formula ${{C}_{7}}{{H}_{14}}$.
- But we need the possible isomers by excluding stereoisomers.
- Therefore the possible cyclopentane structures are four only with molecular formula ${{C}_{7}}{{H}_{14}}$ by excluding stereoisomers.
Note: Stereoisomers are also called as spatial isomers; they have the same molecular formula but different three dimensional arrangements of atoms. Stereoisomers are mirror images and are optically active in nature.
Complete step-by-step answer:- In the question it is asked to draw the structures of cyclopentane that are possible with the molecular formula of ${{C}_{7}}{{H}_{14}}$ by excluding the stereoisomers.
- In the given molecular formula there are seven carbon atoms and fourteen hydrogen atoms are present.
- The basic structure of cyclopentane is as follows.

- Five carbon atoms are going to attached end to end each other.
- Stereoisomers are the isomers that have the same molecular formula and same sequence of bonds but they differ in three dimensional orientations.
- The possible cyclopentane structures with the molecular formula are as follows.

- There are only four possible cyclopentane structures by excluding the stereoisomers with the molecular formula of ${{C}_{7}}{{H}_{14}}$.
- Structure D and B can form two stereoisomers.
- Structure C and A can form one more stereoisomer with the same molecular formula ${{C}_{7}}{{H}_{14}}$.
- But we need the possible isomers by excluding stereoisomers.
- Therefore the possible cyclopentane structures are four only with molecular formula ${{C}_{7}}{{H}_{14}}$ by excluding stereoisomers.
Note: Stereoisomers are also called as spatial isomers; they have the same molecular formula but different three dimensional arrangements of atoms. Stereoisomers are mirror images and are optically active in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




