
$ C{{u}^{2+}}+2{{e}^{-~}}\to Cu $
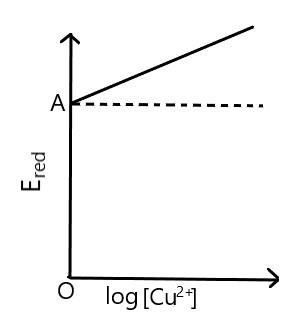
$ Ered{ }Vs.Log\left[ C{{u}^{2+}} \right] $ graph is of the type as shown in figure where $ OA=0.34V $ , then electrode potential of the half-cell of $ CuC{{u}^{2+}}\left( 0.1M \right) $ will be:
(A) $ -0.34+\dfrac{0.0591}{2}V $
(B) $ 0.34+0.0591V $
(C) $ 0.34V $
(D) none of these

Answer
543.6k+ views
Hint :The electrode potential of a cell can be calculated by the use of Nernst equation, which establishes a relationship between the concentration of the ions in the cell as well as the electrode and standard electrode potential of the cell.
However, it also takes in account the number of electrons which are involved in the cell reaction so as to get a more precise value.
$ {{E}_{cell}}=E{{{}^\circ }_{cell}}-\dfrac{0.059}{z}logQ $
Cell potential which is also called the emf of the cell is represented by the symbol $ ~{{E}_{cell}} $ at the same temperature of consideration or in other words constant temperature
Standard cell potential is denoted by $ E{{{}^\circ }_{cell}} $ , The number of electrons which are transferred in the half or full reaction of the cell is denoted by $ ~z $ and the $ Q $ denotes the concentration of the ion.
Complete Step By Step Answer:
In order to answer this question we will consider an equation known as the Nernst equation. The Nernst equation is a type of equation which establishes a relationship between the reduction potential of an electrochemical reaction with the standard electrode potential of the substance involved in the reaction, along with the activities and its temperature, of the chemical species which are observed to be undergoing reduction and oxidation. However, we will be using a modified version of the Nernst equation which will consider the concentration of the copper ions along with the electrode potential of the same.
$ {{E}_{cell}}=E{{{}^\circ }_{cell}}-\dfrac{0.059}{z}logQ $
Cell potential which is also called the emf of the cell is represented by the symbol $ ~{{E}_{cell}} $ at the same temperature of consideration or in other words constant temperature
Standard cell potential is denoted by $ E{{{}^\circ }_{cell}} $ , The number of electrons transferred in the half-reaction or full cell reaction is denoted by $ ~z $ and the $ Q $ denotes the concentration of the ion.
Now, we will write this equation specifically for the copper reaction, which is,
$ {{E}_{Cu/C{{u}^{2+}}}}={{E}_{Cu/C{{u}^{2+}}}}-\dfrac{0.059}{2}log[C{{u}^{2+}}] $
The value of $ ~z $ is two, because there is an exchange of two electrons in the reaction.
Now, from the graph we can see that if $ \log [C{{u}^{2+}}]=0 $ which means $ [C{{u}^{2+}}]=1 $ then we can say that, $ {{E}_{Cu/C{{u}^{2+}}}}={{E}^{o}}_{Cu/C{{u}^{2+}}} $ .
Also the value of electrode potential is provided to us as $ OA=0.34V $ , we could write it as,
$ {{E}^{o}}_{Cu/C{{u}^{2+}}}=-E_{C{{u}^{2+}}/Cu}^{o}=-0.34 $
So, now we will substitute this value in the equation of the Nernst equation, we get,
$ {{E}_{Cu/C{{u}^{2+}}}}=-0.34-\dfrac{0.059}{2}log0.1 $
The value of ‘Q’ is provided to us in the question as $ 0.1M $ and the rest of the values are as it is. So, if we solve the ‘log’ part of this equation we get,
$ {{E}_{Cu/C{{u}^{2+}}}}=-0.34+\dfrac{0.059}{2}V $
Which seems to be our answer. Now, if we consider the options which are provided to us in the question, we can see that option A is our correct option.
So, the correct option would be A.
Note :
The standard electrode potential is the potential of a half reaction which is measured on the basis of the electrode potential of the standard hydrogen electrode.
Standard conditions in which this measurement should be taken are temperature $ 298K $ and the pressure should be unity and the concentration of the electrolyte should be $ 1M $ .
However, it also takes in account the number of electrons which are involved in the cell reaction so as to get a more precise value.
$ {{E}_{cell}}=E{{{}^\circ }_{cell}}-\dfrac{0.059}{z}logQ $
Cell potential which is also called the emf of the cell is represented by the symbol $ ~{{E}_{cell}} $ at the same temperature of consideration or in other words constant temperature
Standard cell potential is denoted by $ E{{{}^\circ }_{cell}} $ , The number of electrons which are transferred in the half or full reaction of the cell is denoted by $ ~z $ and the $ Q $ denotes the concentration of the ion.
Complete Step By Step Answer:
In order to answer this question we will consider an equation known as the Nernst equation. The Nernst equation is a type of equation which establishes a relationship between the reduction potential of an electrochemical reaction with the standard electrode potential of the substance involved in the reaction, along with the activities and its temperature, of the chemical species which are observed to be undergoing reduction and oxidation. However, we will be using a modified version of the Nernst equation which will consider the concentration of the copper ions along with the electrode potential of the same.
$ {{E}_{cell}}=E{{{}^\circ }_{cell}}-\dfrac{0.059}{z}logQ $
Cell potential which is also called the emf of the cell is represented by the symbol $ ~{{E}_{cell}} $ at the same temperature of consideration or in other words constant temperature
Standard cell potential is denoted by $ E{{{}^\circ }_{cell}} $ , The number of electrons transferred in the half-reaction or full cell reaction is denoted by $ ~z $ and the $ Q $ denotes the concentration of the ion.
Now, we will write this equation specifically for the copper reaction, which is,
$ {{E}_{Cu/C{{u}^{2+}}}}={{E}_{Cu/C{{u}^{2+}}}}-\dfrac{0.059}{2}log[C{{u}^{2+}}] $
The value of $ ~z $ is two, because there is an exchange of two electrons in the reaction.
Now, from the graph we can see that if $ \log [C{{u}^{2+}}]=0 $ which means $ [C{{u}^{2+}}]=1 $ then we can say that, $ {{E}_{Cu/C{{u}^{2+}}}}={{E}^{o}}_{Cu/C{{u}^{2+}}} $ .
Also the value of electrode potential is provided to us as $ OA=0.34V $ , we could write it as,
$ {{E}^{o}}_{Cu/C{{u}^{2+}}}=-E_{C{{u}^{2+}}/Cu}^{o}=-0.34 $
So, now we will substitute this value in the equation of the Nernst equation, we get,
$ {{E}_{Cu/C{{u}^{2+}}}}=-0.34-\dfrac{0.059}{2}log0.1 $
The value of ‘Q’ is provided to us in the question as $ 0.1M $ and the rest of the values are as it is. So, if we solve the ‘log’ part of this equation we get,
$ {{E}_{Cu/C{{u}^{2+}}}}=-0.34+\dfrac{0.059}{2}V $
Which seems to be our answer. Now, if we consider the options which are provided to us in the question, we can see that option A is our correct option.
So, the correct option would be A.
Note :
The standard electrode potential is the potential of a half reaction which is measured on the basis of the electrode potential of the standard hydrogen electrode.
Standard conditions in which this measurement should be taken are temperature $ 298K $ and the pressure should be unity and the concentration of the electrolyte should be $ 1M $ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




