
How many covalent bonds can a carbon atom form with neighboring atoms?
Answer
557.4k+ views
Hint: We need to know what are covalent bonds and what are the factors which determine the number of covalent bonds an element can form with other atoms. The attractive force which holds various constituents such as atoms and ions together in different chemical species is called a chemical bond. A covalent bond is a chemical bond that is formed by the sharing of electron pairs between atoms. Carbon atoms belong to group 4 of the periodic table with atomic number 6 whose covalency we are to study.
Complete step by step answer:
We must know that a strict rule is followed by atoms during covalent bonding which is the octet rule. According to this rule, main group elements tend to bond in such a way, either by losing, gaining or sharing electrons such that each atom has 8 electrons in its outermost (valence shell). This craving of elements to satisfy its octet is the main reason for the chemical bonding. The valence electrons can be determined by writing the electronic configuration of the element.
As we know that the electronic configuration of Carbon whose chemical symbol is $C$ and atomic number is $6$ has the electronic configuration $1{s^2}2{s^2}2{p^2}$ . Hence, it is clear that it has $4$ electrons in its valence shell. These 4 electrons can be involved in covalent chemical bonding. Therefore, it can form four covalent bonds with other atoms. The simplest example to show the four covalent bonds which a carbon atom can form with neighboring atoms is methane ($C{H_4}$). The bonding is shown as follows:
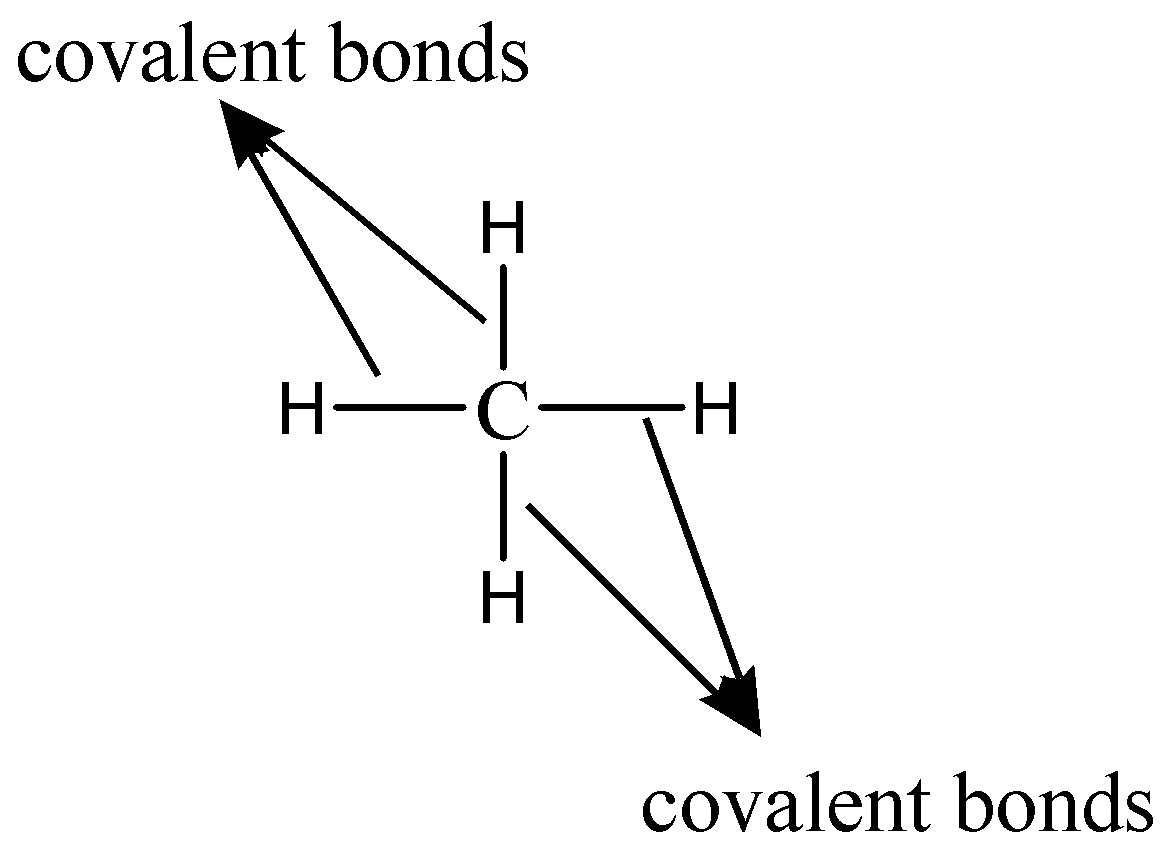
Note:
It must be noted that carbon is called a tetravalent element means it can form four bonds with other atoms. Any of the hydrogen atoms as given in the methane molecule can be replaced with another carbon atom covalently bonded to the first carbon atom. In this way, long and branching chains of carbon compounds can be made which is responsible for the diversity of almost all biological macromolecules.
Complete step by step answer:
We must know that a strict rule is followed by atoms during covalent bonding which is the octet rule. According to this rule, main group elements tend to bond in such a way, either by losing, gaining or sharing electrons such that each atom has 8 electrons in its outermost (valence shell). This craving of elements to satisfy its octet is the main reason for the chemical bonding. The valence electrons can be determined by writing the electronic configuration of the element.
As we know that the electronic configuration of Carbon whose chemical symbol is $C$ and atomic number is $6$ has the electronic configuration $1{s^2}2{s^2}2{p^2}$ . Hence, it is clear that it has $4$ electrons in its valence shell. These 4 electrons can be involved in covalent chemical bonding. Therefore, it can form four covalent bonds with other atoms. The simplest example to show the four covalent bonds which a carbon atom can form with neighboring atoms is methane ($C{H_4}$). The bonding is shown as follows:

Note:
It must be noted that carbon is called a tetravalent element means it can form four bonds with other atoms. Any of the hydrogen atoms as given in the methane molecule can be replaced with another carbon atom covalently bonded to the first carbon atom. In this way, long and branching chains of carbon compounds can be made which is responsible for the diversity of almost all biological macromolecules.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




