
What is the correct order of rate of nitration of the following compounds?
\[\begin{align}
& {{C}_{6}}{{H}_{5}}C{{H}_{3}}\text{, }{{C}_{6}}{{H}_{6}}\text{, }{{C}_{6}}{{D}_{6}}\text{, }{{C}_{6}}{{T}_{6}}\text{, }{{C}_{6}}{{H}_{5}}B{{r}_{3}}\text{, }{{C}_{6}}{{H}_{5}}N{{R}_{3}}\text{, }{{C}_{6}}{{H}_{5}}NM{{e}_{2}} \\
& \text{ A B C D E F G } \\
\end{align}\]
A.G > A > B > C > D > E > F
B.G > B > C > D > A > F
C.G > A > B = C = D > E > F
D.G > A > B > C = D > F > E
Answer
578.1k+ views
Hint: Nitration is an electrophilic substitution reaction. Here, electrophile is, which reacts with the benzene ring. Rate of nitration is found to be directly proportional to the stability of carbocation intermediate.
Complete answer:
- The correct order of nitration will be G > A > B = C = D > E > F, let’s discuss it in detail:
- G.
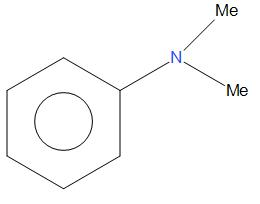
Here, we can say that ${{C}_{6}}{{H}_{5}}NM{{e}_{2}}$ is most stable and will show highest rate of nitration as compared to all other given compounds. This is because there is a strong electron donating group present that will show more +I effect and +R effect, that is there will be more electron density in the ring, due to which it undergoes nitration at a faster rate.
- A.
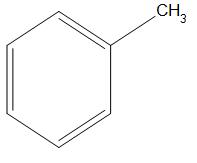
Here, we can see that an electron donating group is present that is $C{{H}_{3}}$ . Which will show +I effect (it is an effect which is shown by those species which have a tendency to donate electrons, present in the carbon chain, and the charge is relayed through the chain and this effect is called +I effect) and will push the electrons. Hence, we can say it will have rate of nitration less than that of ${{C}_{6}}{{H}_{5}}NM{{e}_{2}}$
- B. ${{C}_{6}}{{H}_{6}}$
- C. ${{C}_{6}}{{D}_{6}}$
- D. ${{C}_{6}}{{T}_{_{6}}}$
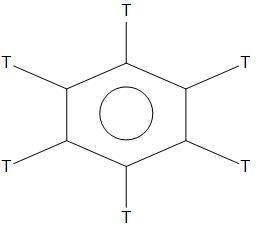
In ${{C}_{6}}{{H}_{6}},{{C}_{6}}{{D}_{6}},{{C}_{6}}{{T}_{_{6}}}$,we can see that there is no +I or +R effect. As there is no side chain is present, hence it is found to have less rate of nitration than that of ${{C}_{6}}{{H}_{5}}NM{{e}_{2}}$and ${{C}_{6}}{{H}_{5}}C{{H}_{3}}$. Hence, in these three compounds the rate of nitration will be equal.
- E. In ${{C}_{6}}{{H}_{6}}B{{r}_{3}}$, there is a halogen Br is present, which will show -I and +R effect. Here, it is found that the +R effect is more dominant. Hence, it will have rate of nitration less than that of ${{C}_{6}}{{H}_{5}}C{{H}_{3}}$
- F. ${{C}_{6}}{{H}_{5}}N{{R}_{3}}$:
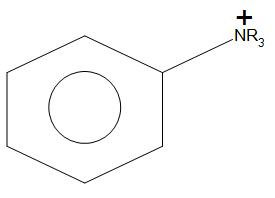
The rate of nitration will be minimal as compared to all other given compounds. This is because it will show –I and –R effect (due to –I effect the electron withdrawing group will start withdrawing electrons from the adjacent carbon atom, and hence this will be no electrons available for donation, due to which rate of nitration will be less) due to which it will destabilise the carbocation.
- Hence, we can conclude that the correct option is (C), that is the correct order of rate of nitration of the given compounds is G > A > B = C = D > E > F.
Note:
- We can say that the electron donating groups in compounds will increase the rate of nitration.
- Compounds that will show + I effect will show the highest rate of nitration. Due to the +I effect the electron donating group will start donating electrons, and hence this will be more electrons available for donation, due to which rate of nitration will be more.
Complete answer:
- The correct order of nitration will be G > A > B = C = D > E > F, let’s discuss it in detail:
- G.

Here, we can say that ${{C}_{6}}{{H}_{5}}NM{{e}_{2}}$ is most stable and will show highest rate of nitration as compared to all other given compounds. This is because there is a strong electron donating group present that will show more +I effect and +R effect, that is there will be more electron density in the ring, due to which it undergoes nitration at a faster rate.
- A.

Here, we can see that an electron donating group is present that is $C{{H}_{3}}$ . Which will show +I effect (it is an effect which is shown by those species which have a tendency to donate electrons, present in the carbon chain, and the charge is relayed through the chain and this effect is called +I effect) and will push the electrons. Hence, we can say it will have rate of nitration less than that of ${{C}_{6}}{{H}_{5}}NM{{e}_{2}}$
- B. ${{C}_{6}}{{H}_{6}}$

- C. ${{C}_{6}}{{D}_{6}}$

- D. ${{C}_{6}}{{T}_{_{6}}}$

In ${{C}_{6}}{{H}_{6}},{{C}_{6}}{{D}_{6}},{{C}_{6}}{{T}_{_{6}}}$,we can see that there is no +I or +R effect. As there is no side chain is present, hence it is found to have less rate of nitration than that of ${{C}_{6}}{{H}_{5}}NM{{e}_{2}}$and ${{C}_{6}}{{H}_{5}}C{{H}_{3}}$. Hence, in these three compounds the rate of nitration will be equal.
- E. In ${{C}_{6}}{{H}_{6}}B{{r}_{3}}$, there is a halogen Br is present, which will show -I and +R effect. Here, it is found that the +R effect is more dominant. Hence, it will have rate of nitration less than that of ${{C}_{6}}{{H}_{5}}C{{H}_{3}}$
- F. ${{C}_{6}}{{H}_{5}}N{{R}_{3}}$:

The rate of nitration will be minimal as compared to all other given compounds. This is because it will show –I and –R effect (due to –I effect the electron withdrawing group will start withdrawing electrons from the adjacent carbon atom, and hence this will be no electrons available for donation, due to which rate of nitration will be less) due to which it will destabilise the carbocation.
- Hence, we can conclude that the correct option is (C), that is the correct order of rate of nitration of the given compounds is G > A > B = C = D > E > F.
Note:
- We can say that the electron donating groups in compounds will increase the rate of nitration.
- Compounds that will show + I effect will show the highest rate of nitration. Due to the +I effect the electron donating group will start donating electrons, and hence this will be more electrons available for donation, due to which rate of nitration will be more.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




