
Correct IUPAC naming is:
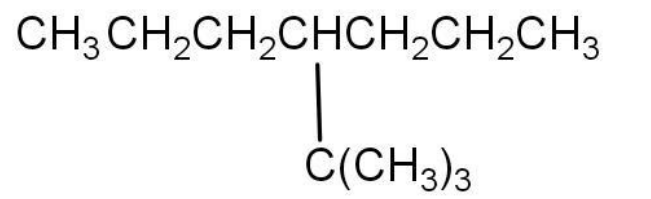
$ A)7\left( {1,1} \right) - \dim ethylethylhep\tan e $
$ B)7\left( {1,1,1} \right) - trimethylethylhep\tan e $
$ C)7\left( {1,1} \right) - \dim ethylethylhep\tan e $
D) None of these

Answer
506.4k+ views
Hint :Alkanes are saturated hydrocarbons consisting of only carbon and hydrogen atoms. The IUPAC nomenclature of alkanes can be written by considering the longest carbon base chain. The root word for the longest carbon chain must be written, the suffix of the carbon chain must end with -ane.
Complete Step By Step Answer:
Given compound contains only carbon and hydrogen atoms. Thus, it is a hydrocarbon. The bonds between carbon and hydrogen are single covalent bonds. Thus, the given compound is an alkane.
The total number of carbon atoms in the given compound is eleven, but four carbons are in the substitution or branched chain.
The longest carbon chain or parent chain is heptane. The $ {4^{th}} $ carbon contains a tertiary butyl group as substitution. Thus, the IUPAC nomenclature can be written as $ 4 - {3^0} - butylhep\tan e $ .
The given options are showing that the substitution is at $ {7^{th}} $ carbon. It is not true, as the substitution is at $ {4^{th}} $ carbon.
Thus, Option D None of these is the correct option.
Additional Information:
Alkanes are also known as paraffins. The bonding in alkanes is single covalent bond, the hybridisation of carbon in alkanes is $ s{p^2} $ . The general molecular formula of alkanes is $ {C_n}{H_{2n + 2}} $ , where n is the number of carbon atoms.
Note :
The suffix of the carbon base chain root word must end with -ane. The root word for seven carbons is hept. The longest carbon chain only must be considered, the substituents must get the lowest number. The substituents must be written in the order of alphabetically.
Complete Step By Step Answer:
Given compound contains only carbon and hydrogen atoms. Thus, it is a hydrocarbon. The bonds between carbon and hydrogen are single covalent bonds. Thus, the given compound is an alkane.
The total number of carbon atoms in the given compound is eleven, but four carbons are in the substitution or branched chain.
The longest carbon chain or parent chain is heptane. The $ {4^{th}} $ carbon contains a tertiary butyl group as substitution. Thus, the IUPAC nomenclature can be written as $ 4 - {3^0} - butylhep\tan e $ .
The given options are showing that the substitution is at $ {7^{th}} $ carbon. It is not true, as the substitution is at $ {4^{th}} $ carbon.
Thus, Option D None of these is the correct option.
Additional Information:
Alkanes are also known as paraffins. The bonding in alkanes is single covalent bond, the hybridisation of carbon in alkanes is $ s{p^2} $ . The general molecular formula of alkanes is $ {C_n}{H_{2n + 2}} $ , where n is the number of carbon atoms.
Note :
The suffix of the carbon base chain root word must end with -ane. The root word for seven carbons is hept. The longest carbon chain only must be considered, the substituents must get the lowest number. The substituents must be written in the order of alphabetically.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a labelled diagram of the human heart and label class 11 biology CBSE

What is 1s 2s 2p 3s 3p class 11 chemistry CBSE




