
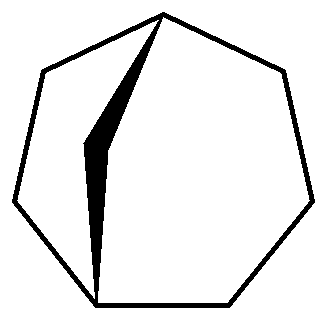
What is the correct IUPAC name of the following compound?
(A) Bicyclo- octane
(B) Tricyclo- octane
(C) Bicyclo[3.2.1]octane
(D) Bicyclo[1.2.3]octane

Answer
559.2k+ views
Hint: According to the question, IUPAC name of this compound which has 8 numbers of carbon bonds. It is a cyclic compound. While naming follow IUPAC nomenclature rules.
Complete answer: According to this question, its option (C) i.e. Bicyclo \[\left[ 3,2,1 \right]\]octane.
First step is to identify the longest carbon chain and give them numbers.
Then count the total number of carbon in this total number of carbon are $8$ with all are single bonds. That means it is a saturated compound. According to saturated compounds, all carbon atoms are connected by single bonds.
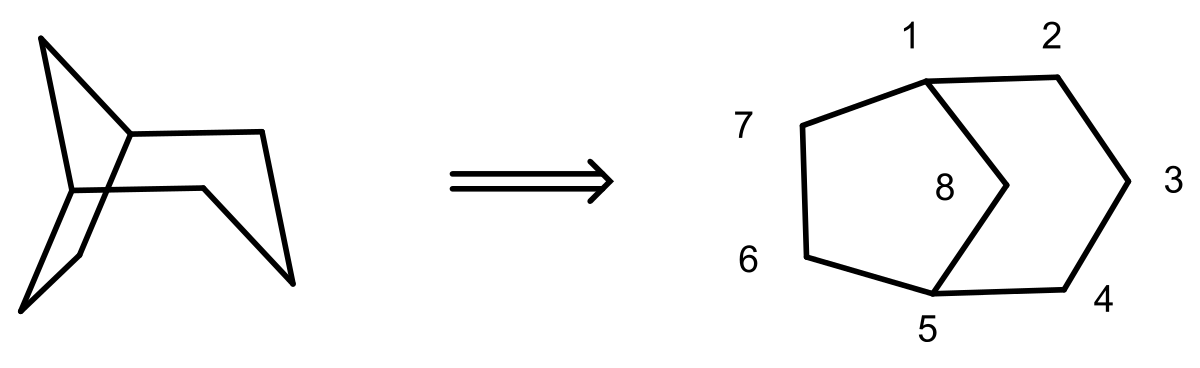
8 carbon atoms and all are in a single bond so it is an octane.
Now we can see that it is forming two ring first ring is $\left[ 1,2,3,4,5,8 \right]$
Second ring is $\left[ 1,7,6,5,8 \right]$ $\left[ 3,2,1 \right]$
Due to these rings it is a bicyclic ring.
So it is bicyclic octane.
Now we have to mention the carbon atom chain excluding $\left[ 1,5 \right]$ carbon atom.
Number of carbons on the two sides of the bridge is denoted in descending order followed by the number of carbons in the bridge.
According to the question, its IUPAC name is Bicyclo$\left[ 3,2,1 \right]$ octane.
Additional information: In this structure Bicyclo is added as a prefix according to the IUPAC nomenclature. Numbering of the carbon chain always begins at one bridgehead atom and follows the carbon chain along the longest path, to the next bridgehead atom. Then numbering is continued along the second longest path. Then these numbers are arranged in descending order.
Note: There are two types of compounds one is saturated compounds second is unsaturated compounds. Saturated compounds are those in which carbon atoms are attached through a single bond whereas unsaturated compounds are those in which carbon atoms are attached through double or triple bonds. Examples of saturated compound are methane and butane etc whereas examples of unsaturated compounds are butane and hexane etc
Complete answer: According to this question, its option (C) i.e. Bicyclo \[\left[ 3,2,1 \right]\]octane.
First step is to identify the longest carbon chain and give them numbers.
Then count the total number of carbon in this total number of carbon are $8$ with all are single bonds. That means it is a saturated compound. According to saturated compounds, all carbon atoms are connected by single bonds.

8 carbon atoms and all are in a single bond so it is an octane.
Now we can see that it is forming two ring first ring is $\left[ 1,2,3,4,5,8 \right]$
Second ring is $\left[ 1,7,6,5,8 \right]$ $\left[ 3,2,1 \right]$
Due to these rings it is a bicyclic ring.
So it is bicyclic octane.
Now we have to mention the carbon atom chain excluding $\left[ 1,5 \right]$ carbon atom.
Number of carbons on the two sides of the bridge is denoted in descending order followed by the number of carbons in the bridge.
According to the question, its IUPAC name is Bicyclo$\left[ 3,2,1 \right]$ octane.
Additional information: In this structure Bicyclo is added as a prefix according to the IUPAC nomenclature. Numbering of the carbon chain always begins at one bridgehead atom and follows the carbon chain along the longest path, to the next bridgehead atom. Then numbering is continued along the second longest path. Then these numbers are arranged in descending order.
Note: There are two types of compounds one is saturated compounds second is unsaturated compounds. Saturated compounds are those in which carbon atoms are attached through a single bond whereas unsaturated compounds are those in which carbon atoms are attached through double or triple bonds. Examples of saturated compound are methane and butane etc whereas examples of unsaturated compounds are butane and hexane etc
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




