
What is correct about saccharin?
A: It is

B: It is 600 times sweeter than sugar
C: It is used as sweetening agent
D: All of these

Answer
586.2k+ views
Hint: Saccharin is an artificial or non-nutritive sweetener that is usually used in the manufacturing of various foods and pharmaceutical products including baked goods, chewing gums, jams, drinks and medicines.
Complete answer:
The molecular structure of saccharin is shown below:
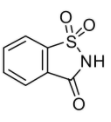
Saccharin is 500 to 600 times sweeter than sucrose (normal sugar). It does not raise blood sugar levels and moreover like all non-nutritive sweeteners, it has no calories or carbs. However, it has a bitter or metallic aftertaste, usually at higher concentrations. This is why saccharin is commonly mixed with other low or zero-calorie sweeteners. For example, saccharin is sometimes integrated with aspartame, another low-calorie sweetener commonly present in carbonated diet drinks. Saccharin is unstable when heated but does not react chemically with other food ingredients, which makes it fit for storage.
Therefore, the correct option is D.
Additional Information Saccharin was first discovered in 1878 by a researcher, Constantin Fahlberg, who was working on coal tar derivatives at that time in a laboratory at the John Hopkins University in Baltimore. Use of the substance became popular during the sugar shortages of World War I.
Note: Saccharin belongs to a class of compounds known as sulfonamides, which can cause allergic reactions in some individuals. These reactions can include headaches, breathing difficulties, diarrhoea and skin problems. However, according to reported literature, the sweetener stevia does not influence blood glucose levels, which makes it a viable option if you are concerned about the possible effects of saccharin.
Complete answer:
The molecular structure of saccharin is shown below:

Saccharin is 500 to 600 times sweeter than sucrose (normal sugar). It does not raise blood sugar levels and moreover like all non-nutritive sweeteners, it has no calories or carbs. However, it has a bitter or metallic aftertaste, usually at higher concentrations. This is why saccharin is commonly mixed with other low or zero-calorie sweeteners. For example, saccharin is sometimes integrated with aspartame, another low-calorie sweetener commonly present in carbonated diet drinks. Saccharin is unstable when heated but does not react chemically with other food ingredients, which makes it fit for storage.
Therefore, the correct option is D.
Additional Information Saccharin was first discovered in 1878 by a researcher, Constantin Fahlberg, who was working on coal tar derivatives at that time in a laboratory at the John Hopkins University in Baltimore. Use of the substance became popular during the sugar shortages of World War I.
Note: Saccharin belongs to a class of compounds known as sulfonamides, which can cause allergic reactions in some individuals. These reactions can include headaches, breathing difficulties, diarrhoea and skin problems. However, according to reported literature, the sweetener stevia does not influence blood glucose levels, which makes it a viable option if you are concerned about the possible effects of saccharin.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




