
What is the coordination number of cobalt in the $ {\left[ {Co{{\left( {en} \right)}_2}B{r_2}} \right]^ + } $ complex?
Answer
490.8k+ views
Hint: To answer the question we need to know the concept of coordination number and coordination bond. A coordinate bond is formed between central metal atoms and ligands. The central metal atom is generally a transition metal atom (d- block series) with vacant d orbital and hence acts as lewis acid. The ligands are atoms or ions that act as lewis bases which donate the electrons to the central metal atom. Coordination number is the number of ligands (i.e. atoms, molecules, and ions) bonded to the central metal atom.
Complete answer:
Let us first discuss the types of ligands before answering the question-
(A)Monodentate ligand-Ligands that bond to the central metal atom only through one site i.e. donates only 1 pair of electrons will be donated. Example- $ C{l^ - }(chloro),B{r^ - }(bromo) $ etc.
(B)Bidentate ligand-Ligands that bond to the central metal atom through 2 donar sites i.e. 2 pairs of electrons will be donated. Example-Ethylene diamine or en or $ N{H_2}C{H_2}C{H_2}N{H_2} $
(C)Polydentate ligand- Ligands that bond to the central metal atom through more than 2 donar sites i.e. more than 2 pairs of electrons will be donated. Example-Ethylene diamine tetraacetate (EDTA).
Now let us look at the coordination complex given in the question-
$ {\left[ {Co{{\left( {en} \right)}_2}B{r_2}} \right]^ + } $
Its name is written as dibromo bis (ethylenediamine) cobalt (III) ion.
The central metal is Cobalt and the ligands are Ethylene diamine and Bromo ligand. Bromo as we have seen above binds through only 1 donor site since it is a Monodentate ligand. So 2 Bromo will bind through 2 donar sites. While ethylene diamine (en) is a bidentate ligand so each will bind through 2 donar sites. Since we have 2 en it will bind through 4 donor sites.
So overall we see that the central metal atom is bonded through 6 donar sites of ligands and hence the coordination number of the complex is 6.
Let us confirm this by looking at the structure of this molecule-
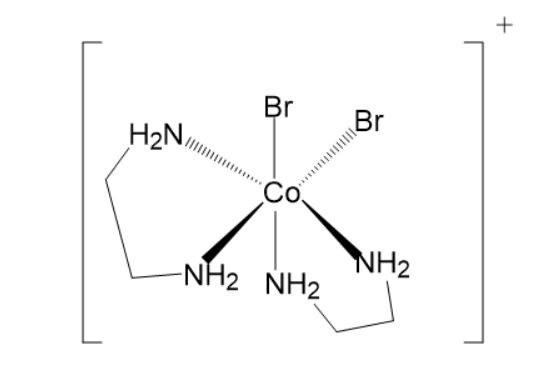
From the diagram, it can be seen that the central atom is bonded to ligands through 6 donor sites.
Note:
It is very interesting to note that in this particular complex the oxidation state of Cobalt is +3. The complex exists in cis (ligands on the same side) and trans- form (ligands on the opposite sides to each other). Like the diagram we have made above is of cis form since both Bromo and both en ligands are on the same side. The cis form of the complex exists in the form of 2 optical isomers (non-superimposable mirror images of each other).
Complete answer:
Let us first discuss the types of ligands before answering the question-
(A)Monodentate ligand-Ligands that bond to the central metal atom only through one site i.e. donates only 1 pair of electrons will be donated. Example- $ C{l^ - }(chloro),B{r^ - }(bromo) $ etc.
(B)Bidentate ligand-Ligands that bond to the central metal atom through 2 donar sites i.e. 2 pairs of electrons will be donated. Example-Ethylene diamine or en or $ N{H_2}C{H_2}C{H_2}N{H_2} $
(C)Polydentate ligand- Ligands that bond to the central metal atom through more than 2 donar sites i.e. more than 2 pairs of electrons will be donated. Example-Ethylene diamine tetraacetate (EDTA).
Now let us look at the coordination complex given in the question-
$ {\left[ {Co{{\left( {en} \right)}_2}B{r_2}} \right]^ + } $
Its name is written as dibromo bis (ethylenediamine) cobalt (III) ion.
The central metal is Cobalt and the ligands are Ethylene diamine and Bromo ligand. Bromo as we have seen above binds through only 1 donor site since it is a Monodentate ligand. So 2 Bromo will bind through 2 donar sites. While ethylene diamine (en) is a bidentate ligand so each will bind through 2 donar sites. Since we have 2 en it will bind through 4 donor sites.
So overall we see that the central metal atom is bonded through 6 donar sites of ligands and hence the coordination number of the complex is 6.
Let us confirm this by looking at the structure of this molecule-

From the diagram, it can be seen that the central atom is bonded to ligands through 6 donor sites.
Note:
It is very interesting to note that in this particular complex the oxidation state of Cobalt is +3. The complex exists in cis (ligands on the same side) and trans- form (ligands on the opposite sides to each other). Like the diagram we have made above is of cis form since both Bromo and both en ligands are on the same side. The cis form of the complex exists in the form of 2 optical isomers (non-superimposable mirror images of each other).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




