
Coordinate bond is present in:
A) Hydronium ion
B) Water
C) Sulphur dioxide
D) Ammonia
Answer
577.8k+ views
Hint: We know that the bond formed between two atoms in which the shared pair of electrons are donated by only one atom but shared by both the atoms is known as the coordinate bond. The coordinate bond is a type of covalent bond.
Complete step by step answer:
Consider the hydronium ion. The molecular formula for the hydronium ion is ${{\text{H}}_{\text{3}}}{{\text{O}}^ + }$. The structure of hydronium ion is as follows:
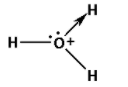
The hydronium ion is formed when the water molecule reacts with a hydrogen ion. The hydrogen ion does not have any electron. The oxygen atom in the water molecule has two lone pairs of electrons.
Thus, the oxygen atom shares one of its lone pairs and forms the coordinate covalent bond.
Thus, the coordinate bond is present in the hydronium ion.
Thus, option (A) is correct.
Consider the water molecule. The molecular formula for the water molecule is ${{\text{H}}_{\text{2}}}{\text{O}}$. The structure of water molecule is as follows:
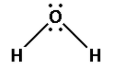
The bonds in the water molecule are formed by the equal sharing of electrons by the hydrogen and oxygen atoms.
Thus, the water molecule has covalent bonds.
Thus, the coordinate bond is absent in the water molecule.
Thus, option (B) is not correct.
Consider the sulphur dioxide molecule. The molecular formula for the sulphur dioxide molecule is ${\text{S}}{{\text{O}}_{\text{2}}}$. The structure of sulphur dioxide molecule is as follows:
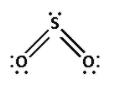
The bonds in the sulphur dioxide molecule are formed by the equal sharing of electrons by the sulphur and oxygen atoms.
Thus, the sulphur dioxide molecule has covalent bonds.
Thus, the coordinate bond is absent in the sulphur dioxide molecule.
Thus, option (C) is not correct.
Consider the ammonia molecule. The molecular formula for the ammonia molecule is ${\text{N}}{{\text{H}}_{\text{3}}}$. The structure of ammonia molecule is as follows:
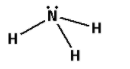
The bonds in the ammonia molecule are formed by the equal sharing of electrons by the nitrogen and hydrogen atoms.
Thus, the ammonia molecule has covalent bonds.
Thus, the coordinate bond is absent in the ammonia molecule.
Thus, option (D) is not correct.
Thus, a coordinate bond is present in hydronium ions.
Thus, the correct option is (A) hydronium ion.
Note: The coordinate bond is also known as the dative bond. The coordinate bond is represented by a single pointed arrow which goes from the atom which shares its lone pair to the other atom.
Complete step by step answer:
Consider the hydronium ion. The molecular formula for the hydronium ion is ${{\text{H}}_{\text{3}}}{{\text{O}}^ + }$. The structure of hydronium ion is as follows:

The hydronium ion is formed when the water molecule reacts with a hydrogen ion. The hydrogen ion does not have any electron. The oxygen atom in the water molecule has two lone pairs of electrons.
Thus, the oxygen atom shares one of its lone pairs and forms the coordinate covalent bond.
Thus, the coordinate bond is present in the hydronium ion.
Thus, option (A) is correct.
Consider the water molecule. The molecular formula for the water molecule is ${{\text{H}}_{\text{2}}}{\text{O}}$. The structure of water molecule is as follows:

The bonds in the water molecule are formed by the equal sharing of electrons by the hydrogen and oxygen atoms.
Thus, the water molecule has covalent bonds.
Thus, the coordinate bond is absent in the water molecule.
Thus, option (B) is not correct.
Consider the sulphur dioxide molecule. The molecular formula for the sulphur dioxide molecule is ${\text{S}}{{\text{O}}_{\text{2}}}$. The structure of sulphur dioxide molecule is as follows:

The bonds in the sulphur dioxide molecule are formed by the equal sharing of electrons by the sulphur and oxygen atoms.
Thus, the sulphur dioxide molecule has covalent bonds.
Thus, the coordinate bond is absent in the sulphur dioxide molecule.
Thus, option (C) is not correct.
Consider the ammonia molecule. The molecular formula for the ammonia molecule is ${\text{N}}{{\text{H}}_{\text{3}}}$. The structure of ammonia molecule is as follows:

The bonds in the ammonia molecule are formed by the equal sharing of electrons by the nitrogen and hydrogen atoms.
Thus, the ammonia molecule has covalent bonds.
Thus, the coordinate bond is absent in the ammonia molecule.
Thus, option (D) is not correct.
Thus, a coordinate bond is present in hydronium ions.
Thus, the correct option is (A) hydronium ion.
Note: The coordinate bond is also known as the dative bond. The coordinate bond is represented by a single pointed arrow which goes from the atom which shares its lone pair to the other atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




