
How will you convert Toluene to Benzaldehyde?
Answer
531.1k+ views
Hint: The conversion of Toluene to Benzaldehyde is used as a laboratory method in order to produce Benzaldehyde.
Complete step by step answer:
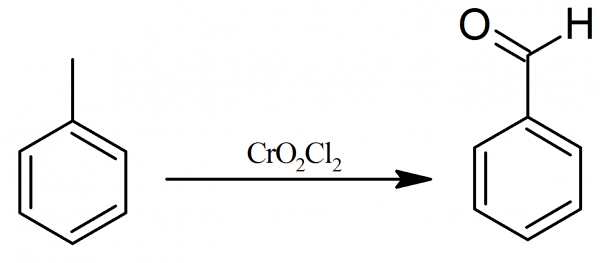
Chromyl chloride dissolved in $\text{C}{{\text{S}}_{\text{2}}}$ or \[\text{CC}{{\text{l}}_{\text{4}}}\] is made to react with toluene in $\text{C}{{\text{S}}_{\text{2}}}$ when a brown coloured product is formed. This product is decomposed with water when benzaldehyde is formed. This is Etard's reaction and is used in the laboratory for preparation of benzaldehyde.
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{C}{{\text{H}}_{\text{3}}}\text{+2Cr}{{\text{O}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\xrightarrow[\text{or C}{{\text{S}}_{\text{2}}}]{\text{CC}{{\text{l}}_{\text{4}}}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{C}{{\text{H}}_{\text{3}}}\cdot \text{2Cr}{{\text{O}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\]
In case, the side-chain contains a group higher than \[\text{C}{{\text{H}}_{\text{3}}}\] group, then end carbon atom of the chain is oxidised by \[\text{Cr}{{\text{O}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\] to \[\text{CHO}\] group.
Benzaldehyde is also formed by oxidation of toluene with chromic oxide in acetic anhydride. Benzaldehyde reacts with acetic anhydride to form benzylidene diacetate which on hydrolysis with alkali or an acid yields benzaldehyde.
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{C}{{\text{H}}_{\text{3}}}\xrightarrow[{{\text{(C}{{\text{H}}_{\text{3}}}\text{CO)}}_{\text{2}}}\text{O}]{\text{Cr}{{\text{O}}_{\text{3}}}\text{, }\!\![\!\!\text{ O }\!\!]\!\!\text{ }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CH(OCOC}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\]
Additional Information:
(i) Benzaldehyde is a colourless oily liquid. Its boiling point is 452K.
(ii) It has the smell of bitter almonds.
(iii) It is sparingly soluble in water but highly soluble in organic solvents.
(iv) It is steam volatile.
(v) It is heavier than water \[\left( \text{sp}\text{. gr}\text{. 1}\text{.0504 at 288K} \right)\]
(vi) It is poisonous in nature.
Note: Benzaldehyde is an organic compound consisting of a benzene ring with a formyl ($-CHO$) substituent. It is the simplest atomic aldehyde and one of the most industrially useful. Benzaldehyde is less reactive than aliphatic aldehyde.
Complete step by step answer:
Chromyl chloride dissolved in $\text{C}{{\text{S}}_{\text{2}}}$ or \[\text{CC}{{\text{l}}_{\text{4}}}\] is made to react with toluene in $\text{C}{{\text{S}}_{\text{2}}}$ when a brown coloured product is formed. This product is decomposed with water when benzaldehyde is formed. This is Etard's reaction and is used in the laboratory for preparation of benzaldehyde.
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{C}{{\text{H}}_{\text{3}}}\text{+2Cr}{{\text{O}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\xrightarrow[\text{or C}{{\text{S}}_{\text{2}}}]{\text{CC}{{\text{l}}_{\text{4}}}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{C}{{\text{H}}_{\text{3}}}\cdot \text{2Cr}{{\text{O}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\]
In case, the side-chain contains a group higher than \[\text{C}{{\text{H}}_{\text{3}}}\] group, then end carbon atom of the chain is oxidised by \[\text{Cr}{{\text{O}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\] to \[\text{CHO}\] group.

Benzaldehyde is also formed by oxidation of toluene with chromic oxide in acetic anhydride. Benzaldehyde reacts with acetic anhydride to form benzylidene diacetate which on hydrolysis with alkali or an acid yields benzaldehyde.
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{C}{{\text{H}}_{\text{3}}}\xrightarrow[{{\text{(C}{{\text{H}}_{\text{3}}}\text{CO)}}_{\text{2}}}\text{O}]{\text{Cr}{{\text{O}}_{\text{3}}}\text{, }\!\![\!\!\text{ O }\!\!]\!\!\text{ }}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CH(OCOC}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\]
Additional Information:
(i) Benzaldehyde is a colourless oily liquid. Its boiling point is 452K.
(ii) It has the smell of bitter almonds.
(iii) It is sparingly soluble in water but highly soluble in organic solvents.
(iv) It is steam volatile.
(v) It is heavier than water \[\left( \text{sp}\text{. gr}\text{. 1}\text{.0504 at 288K} \right)\]
(vi) It is poisonous in nature.
Note: Benzaldehyde is an organic compound consisting of a benzene ring with a formyl ($-CHO$) substituent. It is the simplest atomic aldehyde and one of the most industrially useful. Benzaldehyde is less reactive than aliphatic aldehyde.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




