
How do you convert the following?
(A)Phenol to anisole
(B)Propan-2-ol to 2-methylpropan-2-ol
(C)Aniline to phenol
Answer
577.8k+ views
Hint: For (A): Here to replace (–OH) with (-$OC{H_3}$), first replace H with Na ion and then with ($ - C{H_3}$).
For (B): Here to add ($C{H_3}$) group to the second carbon atom first oxidise the (-OH) group to ketone group then add methyl group using RMgX reagent.
For (C): Here to replace the ($ - N{H_2}$) group with (-OH), ionize amino groups such that it can be easily removed and then react with warm water to provide it with the (-OH) group.
Complete answer:
(A)Phenol to anisole: First let us see the structures of phenol and anisole.
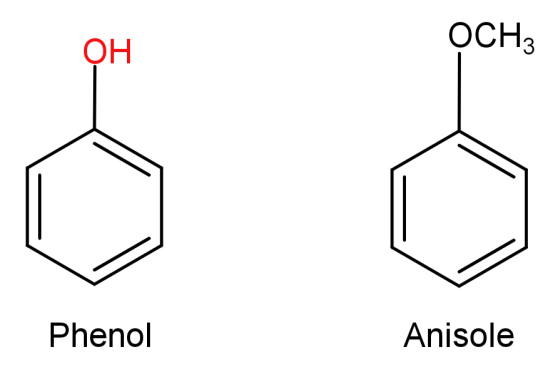
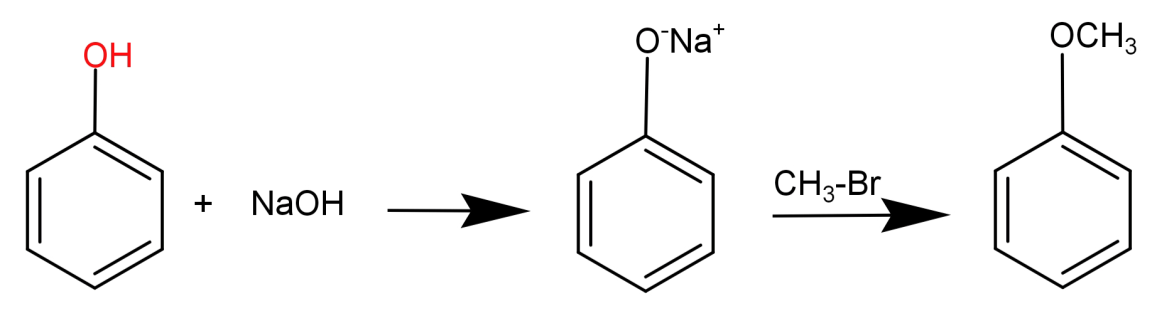
In this what we need to do is replace the H atom from –OH group to $C{H_3}$. This can be done by first reacting phenol with a base NaOH and then treating it with an alkyl halide like $C{H_3} - Br$ .
This can be shown in the form of following reaction:
(B) Propan-2-ol to 2-methylpropan-2-ol: First of all let us see the structures of propan-2-ol and 2-methylpropan-2-ol.
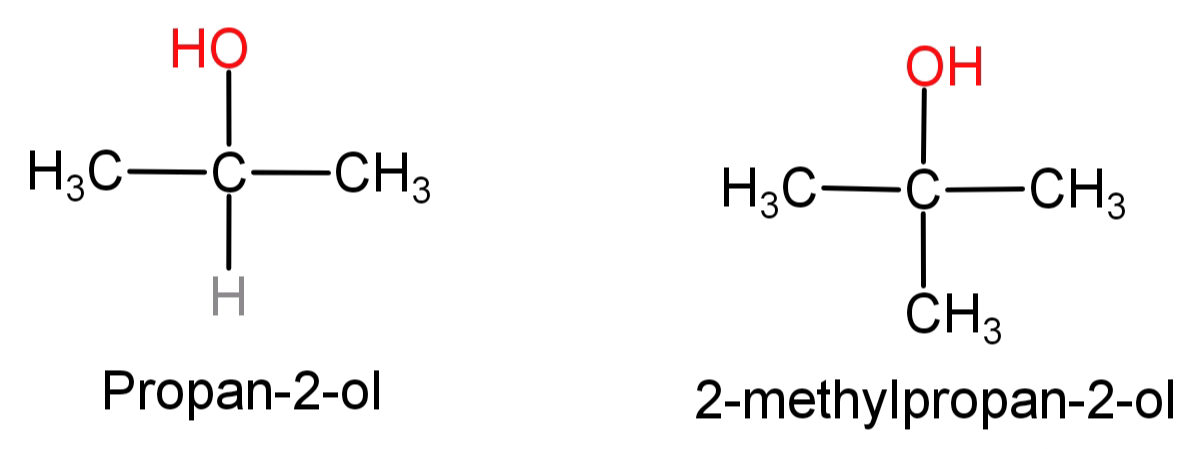
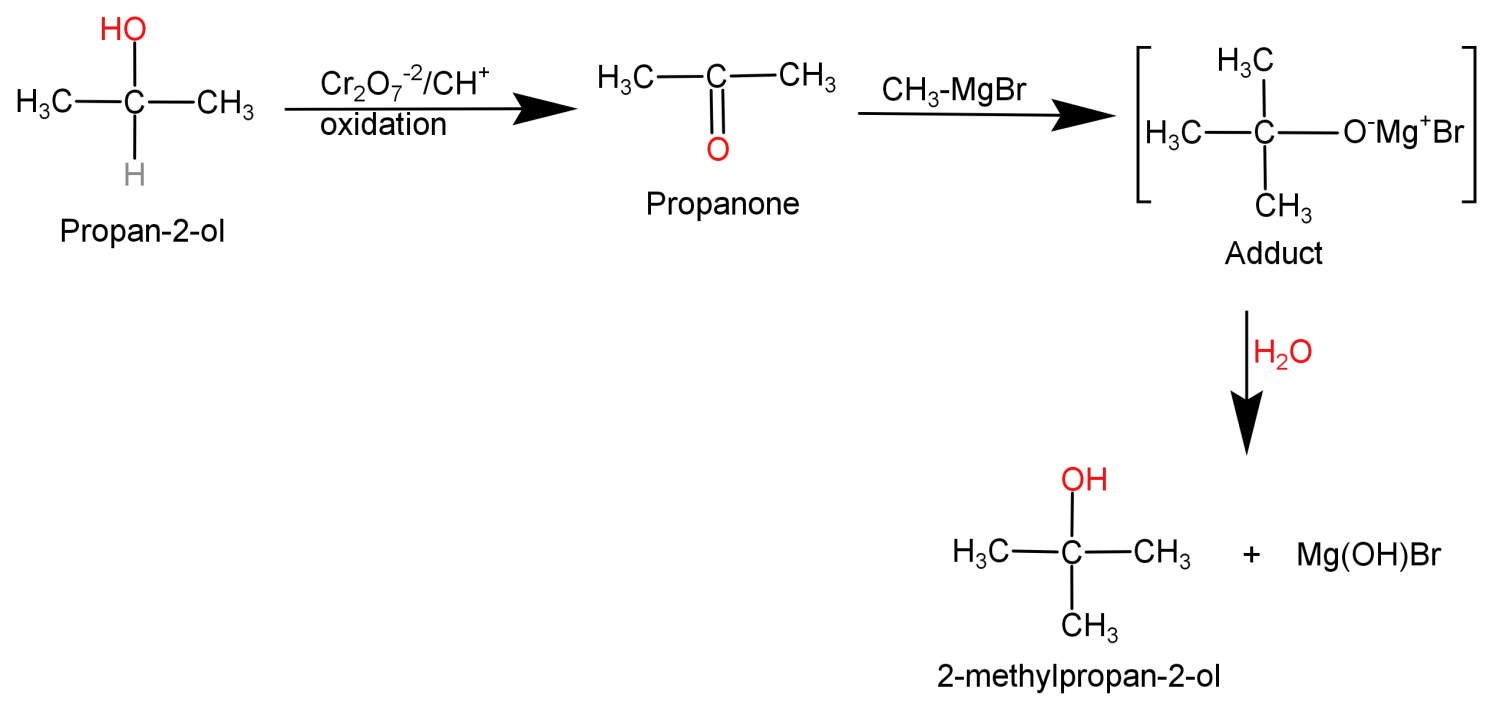
In this what we need to do is replace the H atom from the second carbon with a methyl group or we can say addition of methyl group at second carbon atom. This can be done by simply oxidising the given alcohol using $C{r_2}{O_7}^{ - 2}$ to convert it into a ketone and then adding the methyl group using the Grignard reagent ($C{H_3}MgBr$).
This conversion can be shown in the form of following reaction:
(C)Aniline to phenol: First of all let us see the structures of aniline and phenol.
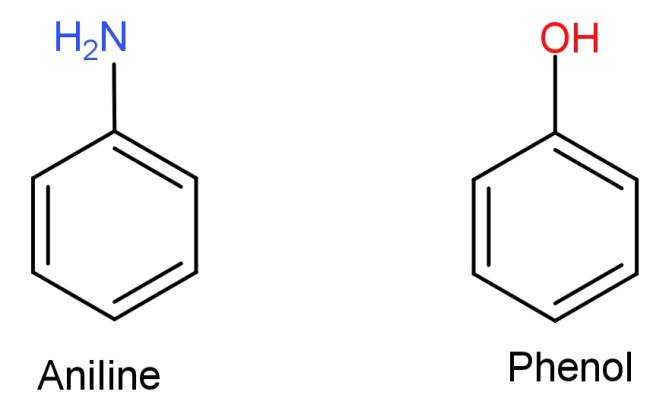
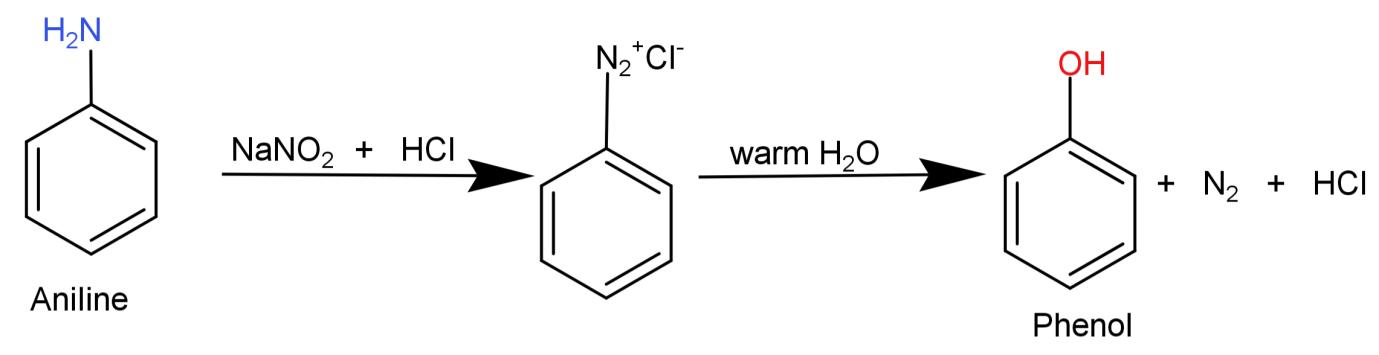
Here what we need to do is replace the $ - N{H_2}$ (amino) group from aniline with –OH (hydroxyl) group. This can be done by simply converting the aniline to diazonium salt so that the amino group can be easily replaced and then reacting it with warm water to replace it with –OH (hydroxyl) group.
This conversion can be shown in the form of following reaction:
Note:
For (A): Sodium phenoxide formed as intermediate is a moderately strong base and thus easily undergoes electrophilic aromatic substitution.
For (B): When reacted with ketones and aldehydes the Grignard reagent usually acts as a base and as nucleophile in nucleophilic aliphatic substitution.
For (C): The diazonium salts are light sensitive and easily break down under UV light. Also they are often very explosive.
For (B): Here to add ($C{H_3}$) group to the second carbon atom first oxidise the (-OH) group to ketone group then add methyl group using RMgX reagent.
For (C): Here to replace the ($ - N{H_2}$) group with (-OH), ionize amino groups such that it can be easily removed and then react with warm water to provide it with the (-OH) group.
Complete answer:
(A)Phenol to anisole: First let us see the structures of phenol and anisole.

In this what we need to do is replace the H atom from –OH group to $C{H_3}$. This can be done by first reacting phenol with a base NaOH and then treating it with an alkyl halide like $C{H_3} - Br$ .
This can be shown in the form of following reaction:

(B) Propan-2-ol to 2-methylpropan-2-ol: First of all let us see the structures of propan-2-ol and 2-methylpropan-2-ol.

In this what we need to do is replace the H atom from the second carbon with a methyl group or we can say addition of methyl group at second carbon atom. This can be done by simply oxidising the given alcohol using $C{r_2}{O_7}^{ - 2}$ to convert it into a ketone and then adding the methyl group using the Grignard reagent ($C{H_3}MgBr$).
This conversion can be shown in the form of following reaction:

(C)Aniline to phenol: First of all let us see the structures of aniline and phenol.

Here what we need to do is replace the $ - N{H_2}$ (amino) group from aniline with –OH (hydroxyl) group. This can be done by simply converting the aniline to diazonium salt so that the amino group can be easily replaced and then reacting it with warm water to replace it with –OH (hydroxyl) group.
This conversion can be shown in the form of following reaction:

Note:
For (A): Sodium phenoxide formed as intermediate is a moderately strong base and thus easily undergoes electrophilic aromatic substitution.
For (B): When reacted with ketones and aldehydes the Grignard reagent usually acts as a base and as nucleophile in nucleophilic aliphatic substitution.
For (C): The diazonium salts are light sensitive and easily break down under UV light. Also they are often very explosive.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




