
How will you convert the Ethanol to formaldehyde?
Answer
583.5k+ views
Hint: Ethanol is an organic compound in which there are two carbon atoms and the functional group $-OH$ is present. Formaldehyde is a compound having one carbon atom having the functional group $-CHO$. So, the two carbon atoms have to be converted into one carbon atom.
Complete step by step solution:
Ethanol is an organic compound in which there are two carbon atoms and the functional group $-OH$ is present and its formula is $C{{H}_{3}}-C{{H}_{2}}OH$.
Formaldehyde is a compound having one carbon atom having the functional group $-CHO$ and its formula is $HCHO$.
So conversion of ethanol to formaldehyde, there are three steps involved. First, we have to convert the ethanol to ethene. And then this ethene is converted into formaldehyde.
The ethanol can be converted into ethene by the action of concentrated sulfuric acid at 433-443 K, this is due to the fact that when primary alcohol is treated with sulfuric acid, the alcohol undergoes intramolecular dehydration and forms alkenes. The reaction is:
$C{{H}_{3}}-C{{H}_{2}}OH\xrightarrow[443K]{{{H}_{2}}S{{O}_{4}}}C{{H}_{2}}=C{{H}_{2}}+{{H}_{2}}O$
Now, this ethene is oxidized to form formaldehyde. This takes place when the ethene is oxidized with ozone. When ethene is treated with ozone the double bond will be attacked and it will convert into 2 moles of formaldehyde. The reaction is given below:
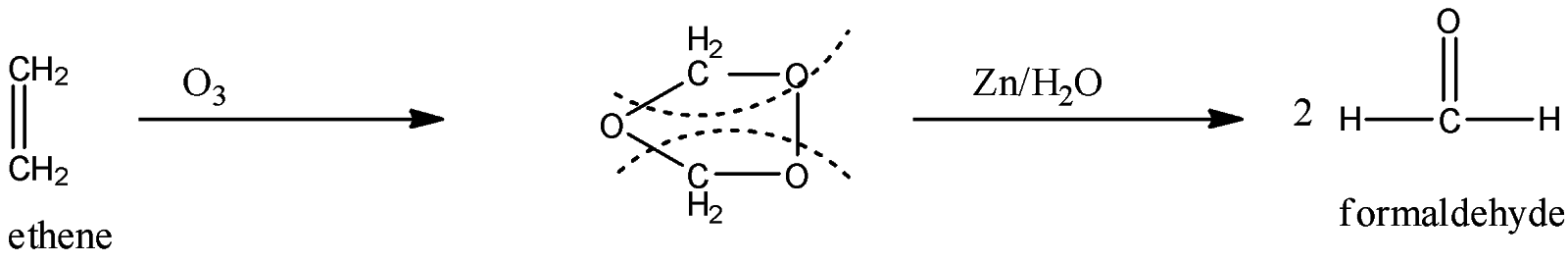
In the above reaction, the solvent must be used like carbon tetrachloride, chloroform, etc at low temperature for the reaction to proceed smoothly.
Note: If propene is used for ozonolysis, there will be the formation of two products i.e., ethanal and methanal. In the ozonolysis, the transition state formed is called Ozonide and it is highly unstable.
Complete step by step solution:
Ethanol is an organic compound in which there are two carbon atoms and the functional group $-OH$ is present and its formula is $C{{H}_{3}}-C{{H}_{2}}OH$.
Formaldehyde is a compound having one carbon atom having the functional group $-CHO$ and its formula is $HCHO$.
So conversion of ethanol to formaldehyde, there are three steps involved. First, we have to convert the ethanol to ethene. And then this ethene is converted into formaldehyde.
The ethanol can be converted into ethene by the action of concentrated sulfuric acid at 433-443 K, this is due to the fact that when primary alcohol is treated with sulfuric acid, the alcohol undergoes intramolecular dehydration and forms alkenes. The reaction is:
$C{{H}_{3}}-C{{H}_{2}}OH\xrightarrow[443K]{{{H}_{2}}S{{O}_{4}}}C{{H}_{2}}=C{{H}_{2}}+{{H}_{2}}O$
Now, this ethene is oxidized to form formaldehyde. This takes place when the ethene is oxidized with ozone. When ethene is treated with ozone the double bond will be attacked and it will convert into 2 moles of formaldehyde. The reaction is given below:

In the above reaction, the solvent must be used like carbon tetrachloride, chloroform, etc at low temperature for the reaction to proceed smoothly.
Note: If propene is used for ozonolysis, there will be the formation of two products i.e., ethanal and methanal. In the ozonolysis, the transition state formed is called Ozonide and it is highly unstable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




