
How will you convert propyne to acetone?
Answer
582.3k+ views
Hint: When the propyne is made to undergo the reaction with the reagents like mercuric sulphate and then followed by its hydrolysis, then the end product of this reaction is the acetone and thus, the propyne is converted. Now, you can easily write the reaction involved in it.
Complete step by step solution:
Propyne is a member belonging to the family of the alkynes which are those hydrocarbons which consist of the triple bond between the carbon atoms and are, thus, also called as the unsaturated hydrocarbon and their general chemical formula is ${{C}_{n}}{{H}_{2n-2}}$ Propyne has three carbon as the name indicates (prop=3 and yne= triple bond) and thus, has the molecular as ${{C}_{3}}{{H}_{5}}$.
On the other hand, acetone is member belonging to the family of the ketone which consists of -C=O group in their compounds and like the propyne, it also consists of the three carbon atoms in it and has the molecular formula as ${{C}_{3}}{{H}_{4}}O$.
Propyne can be converted to the acetone when it is made to undergo the reaction with mercuric sulphate followed by hydrolysis and thus, resultant product, thus formed is acetone. The reaction occurs as:
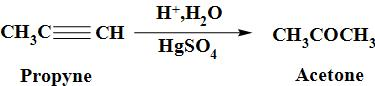
Acetone is a colourless liquid with a pleasant and fruity odour.
Thus, in this way, we can convert propyne into the acetone.
Note: acetone is used in the makeup products i.e. the cosmetics, in foods, can be used to denature certain alcohols, and is a very important laboratory reagent and is used in the production of the many other chemical compounds.
Complete step by step solution:
Propyne is a member belonging to the family of the alkynes which are those hydrocarbons which consist of the triple bond between the carbon atoms and are, thus, also called as the unsaturated hydrocarbon and their general chemical formula is ${{C}_{n}}{{H}_{2n-2}}$ Propyne has three carbon as the name indicates (prop=3 and yne= triple bond) and thus, has the molecular as ${{C}_{3}}{{H}_{5}}$.
On the other hand, acetone is member belonging to the family of the ketone which consists of -C=O group in their compounds and like the propyne, it also consists of the three carbon atoms in it and has the molecular formula as ${{C}_{3}}{{H}_{4}}O$.
Propyne can be converted to the acetone when it is made to undergo the reaction with mercuric sulphate followed by hydrolysis and thus, resultant product, thus formed is acetone. The reaction occurs as:
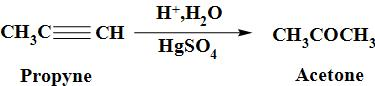
Acetone is a colourless liquid with a pleasant and fruity odour.
Thus, in this way, we can convert propyne into the acetone.
Note: acetone is used in the makeup products i.e. the cosmetics, in foods, can be used to denature certain alcohols, and is a very important laboratory reagent and is used in the production of the many other chemical compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




