
Convert nitrobenzene into phenol.
Answer
597.9k+ views
Hint: To answer this question draw the structure of both the chemical compounds and then figure out the conversion of the nitro group present on benzene to amine group and then an alcohol group. Now you can easily give the answer to this question.
Complete step by step solution: Nitrobenzene organic conversation to phenol is a very important conversion in organic chemistry.
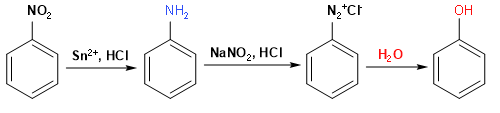
Here we will first convert nitrobenzene to aniline by a reduction reaction and then we will convert aniline to benzene diazonium chloride using $NaNO_{ 2 }$/HCl. Finally, we just need to add some water in reaction to synthesize our final product phenol.
Conversion of nitrobenzene to aniline -
In this step, nitrobenzene is reduced by using Sn and concentrated HCl to form aniline. It reduces the $NO_{ 2 }$ group to $NH_{ 2 }$ and forms the chemical compound Aniline.
Conversion of aniline to phenol -
In this step, aniline is treated with $NaNO_{ 2 }$ and HCl in cold conditions to form benzene diazonium chloride, which on further treatment with water gives phenol.
 Therefore, we converted nitrobenzene into phenol.
Therefore, we converted nitrobenzene into phenol.
Note: Let’s look at some of the properties of both of these compounds -
Nitrobenzene is a yellowish, oily, aromatic nitro-compound with an almond-like odor that emits toxic fumes of nitrogen oxides upon combustion. Nitrobenzene is primarily used in the manufacture of aniline.
Phenols are widely used in household products and as intermediates for industrial synthesis. For example, phenol itself is used (in low concentrations) as a disinfectant in household cleaners and in mouthwash. Phenol may have been the first surgical antiseptic.
Complete step by step solution: Nitrobenzene organic conversation to phenol is a very important conversion in organic chemistry.
Here we will first convert nitrobenzene to aniline by a reduction reaction and then we will convert aniline to benzene diazonium chloride using $NaNO_{ 2 }$/HCl. Finally, we just need to add some water in reaction to synthesize our final product phenol.
Conversion of nitrobenzene to aniline -
In this step, nitrobenzene is reduced by using Sn and concentrated HCl to form aniline. It reduces the $NO_{ 2 }$ group to $NH_{ 2 }$ and forms the chemical compound Aniline.
Conversion of aniline to phenol -
In this step, aniline is treated with $NaNO_{ 2 }$ and HCl in cold conditions to form benzene diazonium chloride, which on further treatment with water gives phenol.

Note: Let’s look at some of the properties of both of these compounds -
Nitrobenzene is a yellowish, oily, aromatic nitro-compound with an almond-like odor that emits toxic fumes of nitrogen oxides upon combustion. Nitrobenzene is primarily used in the manufacture of aniline.
Phenols are widely used in household products and as intermediates for industrial synthesis. For example, phenol itself is used (in low concentrations) as a disinfectant in household cleaners and in mouthwash. Phenol may have been the first surgical antiseptic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




