
How will you convert ethanoic acid into benzene?
Answer
526.6k+ views
Hint: The ethanoic acid cannot be converted into benzene by one or two steps. Total nine steps are involved in the conversion of ethanoic acid to benzene. The main reactions involved are chlorination, bromination, Wurtz reaction.
Complete step by step answer:
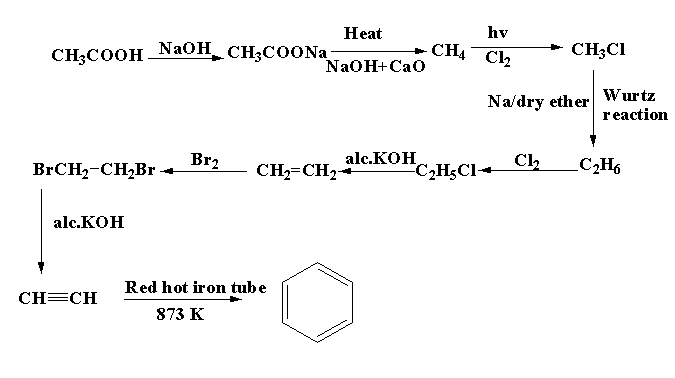
For converting ethanoic acid $C{H_3}COOH$ to benzene ${C_6}{H_6}$ , a number of steps are involved. First the ethanoic acid is reacted with base sodium hydroxide NaOH, the product formed is sodium acetate $C{H_3}COONa$. After that, the sodium acetate is heated with sodium hydroxide and calcium oxide, methane $C{H_4}$ is formed as the main product and sodium carbonate$N{a_2}C{O_3}$ is formed as the by product. The methane is then reacted with chlorine in presence of light to form methyl chloride $C{H_3}Cl$. The methyl chloride is then reacted with sodium metal in dry ether to form ethane ${C_2}{H_6}$. The reaction is known as Wurtz reaction. The ethane then undergo chlorination to form ethyl chloride \[{C_2}{H_5}Cl\]
. The methyl chloride is then reacted with alcoholic potassium hydroxide to form ethene $C{H_2} = C{H_2}$. The ethene then undergoes bromination to form 1,2-dibromoethane. $BrC{H_2} - C{H_2}Br$. The compound 1,2-dibromoethane is treated with alcoholic potassium hydroxide to form ethyne. Ethyne is when passed through a red hot iron tube at 873 K, benzene ${C_6}{H_6}$ is formed.
The reaction scheme for the preparation of benzene from ethanoic acid is shown below.
Note:
Make sure that the potassium hydroxide used in the reaction is alcoholic in nature, if aqueous potassium hydroxide is used instead of alcoholic potassium hydroxide then the product formed will be alcohol. The alcoholic potassium hydroxide is prepared by mixing potassium hydroxide solution with ethanol.
Complete step by step answer:
For converting ethanoic acid $C{H_3}COOH$ to benzene ${C_6}{H_6}$ , a number of steps are involved. First the ethanoic acid is reacted with base sodium hydroxide NaOH, the product formed is sodium acetate $C{H_3}COONa$. After that, the sodium acetate is heated with sodium hydroxide and calcium oxide, methane $C{H_4}$ is formed as the main product and sodium carbonate$N{a_2}C{O_3}$ is formed as the by product. The methane is then reacted with chlorine in presence of light to form methyl chloride $C{H_3}Cl$. The methyl chloride is then reacted with sodium metal in dry ether to form ethane ${C_2}{H_6}$. The reaction is known as Wurtz reaction. The ethane then undergo chlorination to form ethyl chloride \[{C_2}{H_5}Cl\]
. The methyl chloride is then reacted with alcoholic potassium hydroxide to form ethene $C{H_2} = C{H_2}$. The ethene then undergoes bromination to form 1,2-dibromoethane. $BrC{H_2} - C{H_2}Br$. The compound 1,2-dibromoethane is treated with alcoholic potassium hydroxide to form ethyne. Ethyne is when passed through a red hot iron tube at 873 K, benzene ${C_6}{H_6}$ is formed.
The reaction scheme for the preparation of benzene from ethanoic acid is shown below.

Note:
Make sure that the potassium hydroxide used in the reaction is alcoholic in nature, if aqueous potassium hydroxide is used instead of alcoholic potassium hydroxide then the product formed will be alcohol. The alcoholic potassium hydroxide is prepared by mixing potassium hydroxide solution with ethanol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




