
Convert ethane to butane?
Answer
598.2k+ views
Hint: we must have to remember that the Molecular formula of ethane is $C_2$$H_6$ and butane is $C_4$$H_10$
Complete step by step answer:
Let's start with the discussion of ethane and butane, both ethane (prefix eth means 2 carbons) and butane (prefix but means 4 carbons) are alkanes with molecular formulas C2H6 and C4H10¬ respectively. Being an alkane means that both of these hydrocarbons will have no present unsaturation, therefore, no double or triple bonds are present.
Let’s draw the molecular structure of both ethane and butane for better understanding.
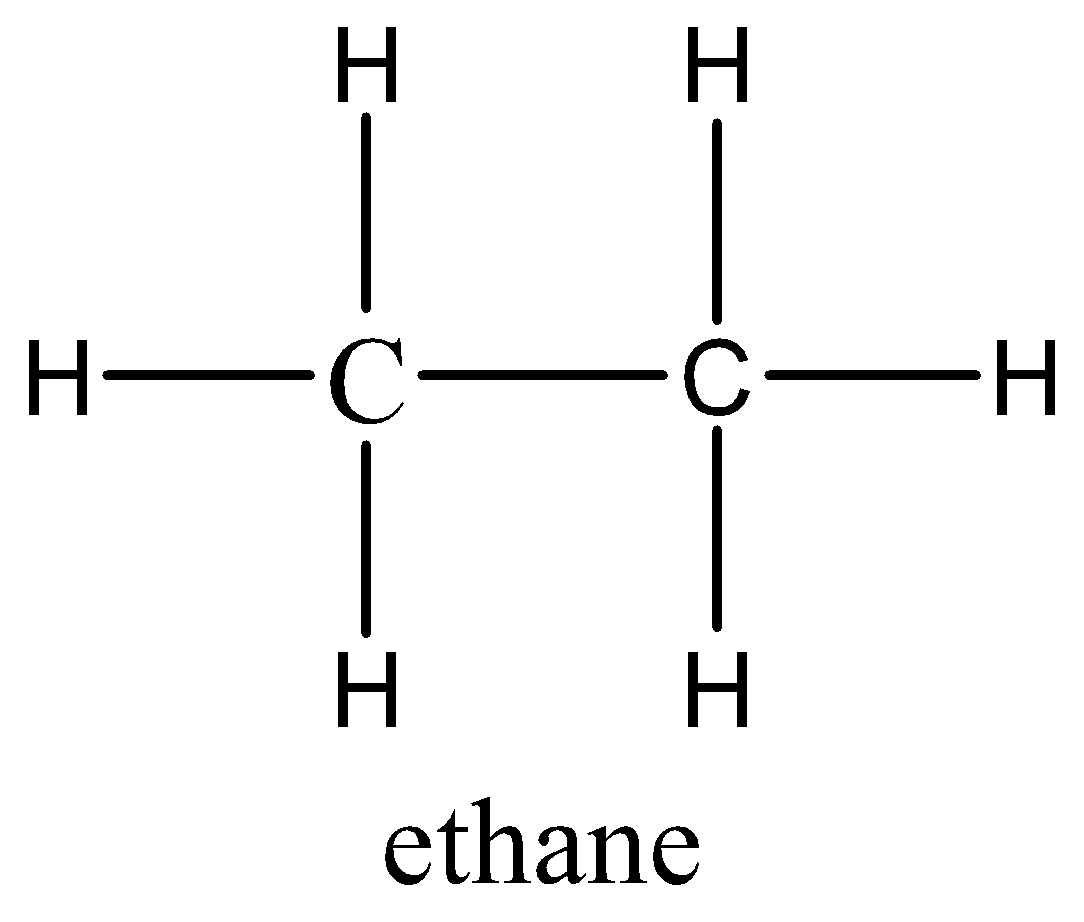
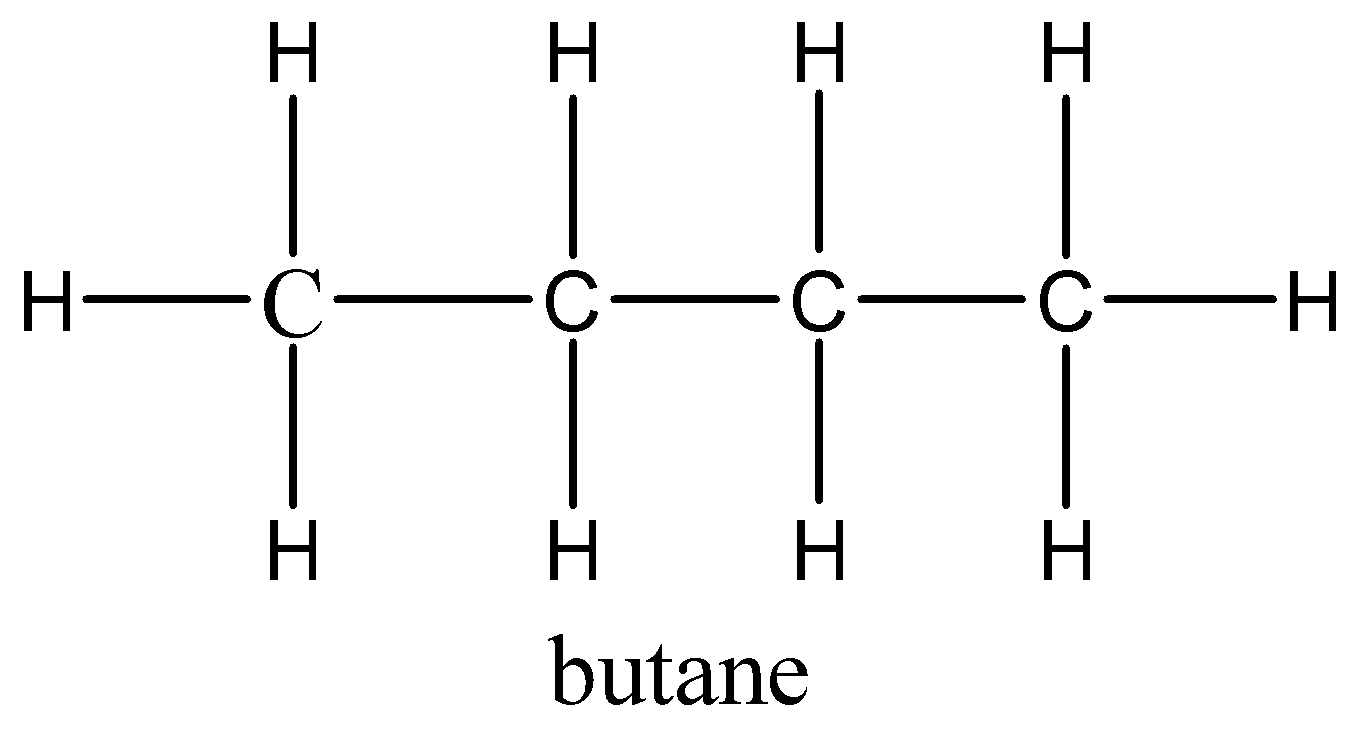
So, we have both the structures and by seeing, and analysing both we get that the difference between both the alkanes is only the no. of carbons and no. of hydrogen.
So, if we take 2 ethanes we get the same no. of carbons but 2 extra hydrogens. But, when we react with these two ethane and combine them to form a single bond between these two, the two hydrogens will be removed and we will get the butane.
So, how will this reaction proceed? Well, this reaction will proceed through Wurtz Reaction.
Wurtz Reaction is the reaction in which two lower alkyl halides combine in the presence of alkali metal or dry ether medium to form a higher alkane.
So first, the ethane will react with halogen which might be bromine to give alkyl halide
\[{\text{C}}{{\text{H}}_3}{\text{ - C}}{{\text{H}}_3}{\text{ + B}}{{\text{r}}_2}\xrightarrow{{{\text{sunlight}}}}{\text{ C}}{{\text{H}}_3}{\text{ - C}}{{\text{H}}_2}{\text{Br + HBr}}\]
Now we have ethyl bromide, now for a second we will take two ethyl bromides and react them in the presence of sodium metal to get butane.
${\text{C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2}{\text{Br + C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2}{\text{Br }}\xrightarrow{{{\text{anhydrous Na}}}}{\text{ C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2} - {\text{C}}{{\text{H}}_2} - {\text{C}}{{\text{H}}_3} + {\text{NaBr}}$
So like this, we will convert the ethane to butane using Wurtz Reaction.
Note: While solving the questions based on Wurtz Reaction the student must keep in mind that one should always use halogens in the presence of sunlight in Wurtz reaction and to use anhydrous Na or ether for the second step.
Complete step by step answer:
Let's start with the discussion of ethane and butane, both ethane (prefix eth means 2 carbons) and butane (prefix but means 4 carbons) are alkanes with molecular formulas C2H6 and C4H10¬ respectively. Being an alkane means that both of these hydrocarbons will have no present unsaturation, therefore, no double or triple bonds are present.
Let’s draw the molecular structure of both ethane and butane for better understanding.


So, we have both the structures and by seeing, and analysing both we get that the difference between both the alkanes is only the no. of carbons and no. of hydrogen.
So, if we take 2 ethanes we get the same no. of carbons but 2 extra hydrogens. But, when we react with these two ethane and combine them to form a single bond between these two, the two hydrogens will be removed and we will get the butane.
So, how will this reaction proceed? Well, this reaction will proceed through Wurtz Reaction.
Wurtz Reaction is the reaction in which two lower alkyl halides combine in the presence of alkali metal or dry ether medium to form a higher alkane.
So first, the ethane will react with halogen which might be bromine to give alkyl halide
\[{\text{C}}{{\text{H}}_3}{\text{ - C}}{{\text{H}}_3}{\text{ + B}}{{\text{r}}_2}\xrightarrow{{{\text{sunlight}}}}{\text{ C}}{{\text{H}}_3}{\text{ - C}}{{\text{H}}_2}{\text{Br + HBr}}\]
Now we have ethyl bromide, now for a second we will take two ethyl bromides and react them in the presence of sodium metal to get butane.
${\text{C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2}{\text{Br + C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2}{\text{Br }}\xrightarrow{{{\text{anhydrous Na}}}}{\text{ C}}{{\text{H}}_3} - {\text{C}}{{\text{H}}_2} - {\text{C}}{{\text{H}}_2} - {\text{C}}{{\text{H}}_3} + {\text{NaBr}}$
So like this, we will convert the ethane to butane using Wurtz Reaction.
Note: While solving the questions based on Wurtz Reaction the student must keep in mind that one should always use halogens in the presence of sunlight in Wurtz reaction and to use anhydrous Na or ether for the second step.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




