
Convert ethanal to isopropyl alcohol.
Answer
572.1k+ views
Hint:Before moving to the steps involved in the conversion of ethanal to isopropyl alcohol, let’s know about both of the compounds. Ethanal is an aldehyde having two carbon atoms since it is ended with the term “al” and starting with the term “eth”. Isopropyl alcohol is a secondary alcohol with a total of three carbon atoms.
Complete answer:
The compounds which have a $ - {{CHO}}$ group are called aldehydes. Carbon is double bonded to oxygen, singly bonded to ${{R}}$ group and hydrogen. ${{R}}$ group may be hydrogen, alkyl group or any aryl group. Alcohol is a compound in which a hydroxyl group is attached to a carbon atom. As we have said that isopropyl alcohol is a secondary alcohol. Secondary alcohol is the alcohol in which a hydroxyl group is attached to a carbon atom which is further attached to other two carbon atoms.
The steps involved in the reaction are given below:
Ethanal is a compound which has two carbon atoms and the product isopropyl alcohol has three carbon atoms. This is made by reacting ethanal with Grignard reagent. The chemical formula of Grignard reagent is ${{RMgBr}}$, where ${{R}}$ is methyl group. From this Grignard reagent, one carbon is added to ethanal molecules.
The chemical reaction is given below:
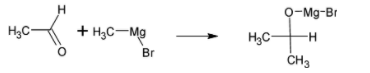
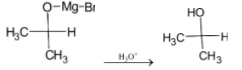
The intermediate undergoes hydrolysis to give isopropyl alcohol. The reaction is given below:
Note:
We can also convert the isopropyl alcohol to a ketone group. Any oxidizing agents like potassium dichromate, sodium dichromate, potassium permanganate or pyridinium chlorochromate can be used to convert to ketone. Propanone is obtained using this reaction.
Complete answer:
The compounds which have a $ - {{CHO}}$ group are called aldehydes. Carbon is double bonded to oxygen, singly bonded to ${{R}}$ group and hydrogen. ${{R}}$ group may be hydrogen, alkyl group or any aryl group. Alcohol is a compound in which a hydroxyl group is attached to a carbon atom. As we have said that isopropyl alcohol is a secondary alcohol. Secondary alcohol is the alcohol in which a hydroxyl group is attached to a carbon atom which is further attached to other two carbon atoms.
The steps involved in the reaction are given below:
Ethanal is a compound which has two carbon atoms and the product isopropyl alcohol has three carbon atoms. This is made by reacting ethanal with Grignard reagent. The chemical formula of Grignard reagent is ${{RMgBr}}$, where ${{R}}$ is methyl group. From this Grignard reagent, one carbon is added to ethanal molecules.
The chemical reaction is given below:

The intermediate undergoes hydrolysis to give isopropyl alcohol. The reaction is given below:

Note:
We can also convert the isopropyl alcohol to a ketone group. Any oxidizing agents like potassium dichromate, sodium dichromate, potassium permanganate or pyridinium chlorochromate can be used to convert to ketone. Propanone is obtained using this reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




