
How will you convert benzamide to aniline?
Answer
573.3k+ views
Hint: Primary amines can be prepared from amides by treatment with $\text{ B}{{\text{r}}_{\text{2}}}\text{ }$ and $\text{ KOH }$ or $\text{ NaOH }$. The amines formed contain one carbon atom less than the parent amide. Therefore, this method is used for stepping down the series in organic conversions. This is known as the Hoffman bromamide reaction. The general reaction is as depicted below,
$\text{ }\begin{matrix}
\text{RCON}{{\text{H}}_{\text{2}}} & \text{+} & \text{B}{{\text{r}}_{\text{2}}} & \text{+} & \text{4KOH} & \to & \text{RN}{{\text{H}}_{\text{2}}} & \text{+} & {{\text{K}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}} & \text{+} & \text{2KBr} & \text{+} & \text{2}{{\text{H}}_{\text{2}}}\text{O} \\
\text{Amide} & {} & {} & {} & {} & {} & {{1}^{\text{o}}}-\text{Amine} & {} & {} & {} & {} & {} & {} \\
\end{matrix}\text{ }$
Complete answer:
When amide is treated with the bromine in the ethanolic solution of sodium hydroxide or potassium hydroxide, amide undergoes degradation to form an amine which contains one carbon atom less than the parent amide. Benzamide is an amide. It undergoes the benzamide degradation reaction. In this reaction, the benzamide is treated with the bromine in presence of sodium hydroxide or potassium hydroxide. The general reaction for the conversion of benzamide to aniline is as shown below,
$\text{ }\begin{matrix}
{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CON}{{\text{H}}_{\text{2}}} & \text{+} & \text{B}{{\text{r}}_{\text{2}}} & \text{+} & \text{4KOH} & \to & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{N}{{\text{H}}_{\text{2}}} & \text{+} & {{\text{K}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}} & \text{+} & \text{2KBr} & \text{+} & \text{2}{{\text{H}}_{\text{2}}}\text{O} \\
\text{Benzamide} & {} & {} & {} & {} & {} & \text{Aniline} & {} & {} & {} & {} & {} & {} \\
\end{matrix}\text{ }$
The general mechanism for the conversion of benzamide to aniline is as shown below,
1. Base such as potassium hydroxide and sodium hydroxide attacks on the amide. Base abstracts protons from benzamide.
2. In the next step the anion reacts with the bromine molecule. This generates N-bromobenzamide.This is an alpha substitution reaction.
3. Next base molecule abstracts a proton from the amide group to generate a bromamide anion.
4. Fourth step involves the rearrangement of bromoimide anion such that the benzyl $\text{ Ar }$ group migrates to the nitrogen atom. This leads to the formation of isocyanate.
5. In the next step water molecules are added to the isocyanate to form carbamic acid. On heating carbamic acid loses carbon dioxide and leads to the formation of primary amine i.e. Aniline.BThe mechanistic pathways for benzamide to aniline are as shown below,
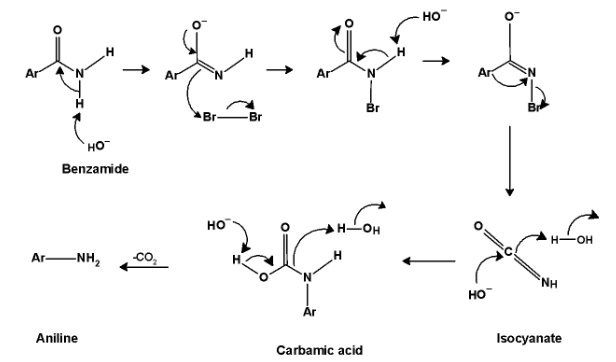
Like this, we have to convert benzamide into aniline.
Note: It may be noted that primary amines can be obtained from amides either by reduction with $\text{ LiAl}{{\text{H}}_{\text{4}}}\text{ }$ or by treating with $\text{ B}{{\text{r}}_{\text{2 }}}$ and base. The reduction with $\text{ LiAl}{{\text{H}}_{\text{4}}}\text{ }$gives amine having the same number of C-atoms as the original amide while reduction with $\text{ NaOH }$ and $\text{ B}{{\text{r}}_{\text{2 }}}$ gives amine having one carbon atom less than the original amide.
$\text{ }\begin{matrix}
\text{RCON}{{\text{H}}_{\text{2}}} & \text{+} & \text{B}{{\text{r}}_{\text{2}}} & \text{+} & \text{4KOH} & \to & \text{RN}{{\text{H}}_{\text{2}}} & \text{+} & {{\text{K}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}} & \text{+} & \text{2KBr} & \text{+} & \text{2}{{\text{H}}_{\text{2}}}\text{O} \\
\text{Amide} & {} & {} & {} & {} & {} & {{1}^{\text{o}}}-\text{Amine} & {} & {} & {} & {} & {} & {} \\
\end{matrix}\text{ }$
Complete answer:
When amide is treated with the bromine in the ethanolic solution of sodium hydroxide or potassium hydroxide, amide undergoes degradation to form an amine which contains one carbon atom less than the parent amide. Benzamide is an amide. It undergoes the benzamide degradation reaction. In this reaction, the benzamide is treated with the bromine in presence of sodium hydroxide or potassium hydroxide. The general reaction for the conversion of benzamide to aniline is as shown below,
$\text{ }\begin{matrix}
{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{CON}{{\text{H}}_{\text{2}}} & \text{+} & \text{B}{{\text{r}}_{\text{2}}} & \text{+} & \text{4KOH} & \to & {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}\text{N}{{\text{H}}_{\text{2}}} & \text{+} & {{\text{K}}_{\text{2}}}\text{C}{{\text{O}}_{\text{3}}} & \text{+} & \text{2KBr} & \text{+} & \text{2}{{\text{H}}_{\text{2}}}\text{O} \\
\text{Benzamide} & {} & {} & {} & {} & {} & \text{Aniline} & {} & {} & {} & {} & {} & {} \\
\end{matrix}\text{ }$
The general mechanism for the conversion of benzamide to aniline is as shown below,
1. Base such as potassium hydroxide and sodium hydroxide attacks on the amide. Base abstracts protons from benzamide.
2. In the next step the anion reacts with the bromine molecule. This generates N-bromobenzamide.This is an alpha substitution reaction.
3. Next base molecule abstracts a proton from the amide group to generate a bromamide anion.
4. Fourth step involves the rearrangement of bromoimide anion such that the benzyl $\text{ Ar }$ group migrates to the nitrogen atom. This leads to the formation of isocyanate.
5. In the next step water molecules are added to the isocyanate to form carbamic acid. On heating carbamic acid loses carbon dioxide and leads to the formation of primary amine i.e. Aniline.BThe mechanistic pathways for benzamide to aniline are as shown below,

Like this, we have to convert benzamide into aniline.
Note: It may be noted that primary amines can be obtained from amides either by reduction with $\text{ LiAl}{{\text{H}}_{\text{4}}}\text{ }$ or by treating with $\text{ B}{{\text{r}}_{\text{2 }}}$ and base. The reduction with $\text{ LiAl}{{\text{H}}_{\text{4}}}\text{ }$gives amine having the same number of C-atoms as the original amide while reduction with $\text{ NaOH }$ and $\text{ B}{{\text{r}}_{\text{2 }}}$ gives amine having one carbon atom less than the original amide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




