
Convert
(a) Phenol to acetophenone
(b) Toluene to m-nitrobenzene
Answer
569.4k+ views
Hint: Synthesis of acetophenone from phenol and synthesis of m-nitro benzene from toluene is carried out through the formation of benzene only. In the synthesis of both the compounds benzene is going to form as an intermediate product.
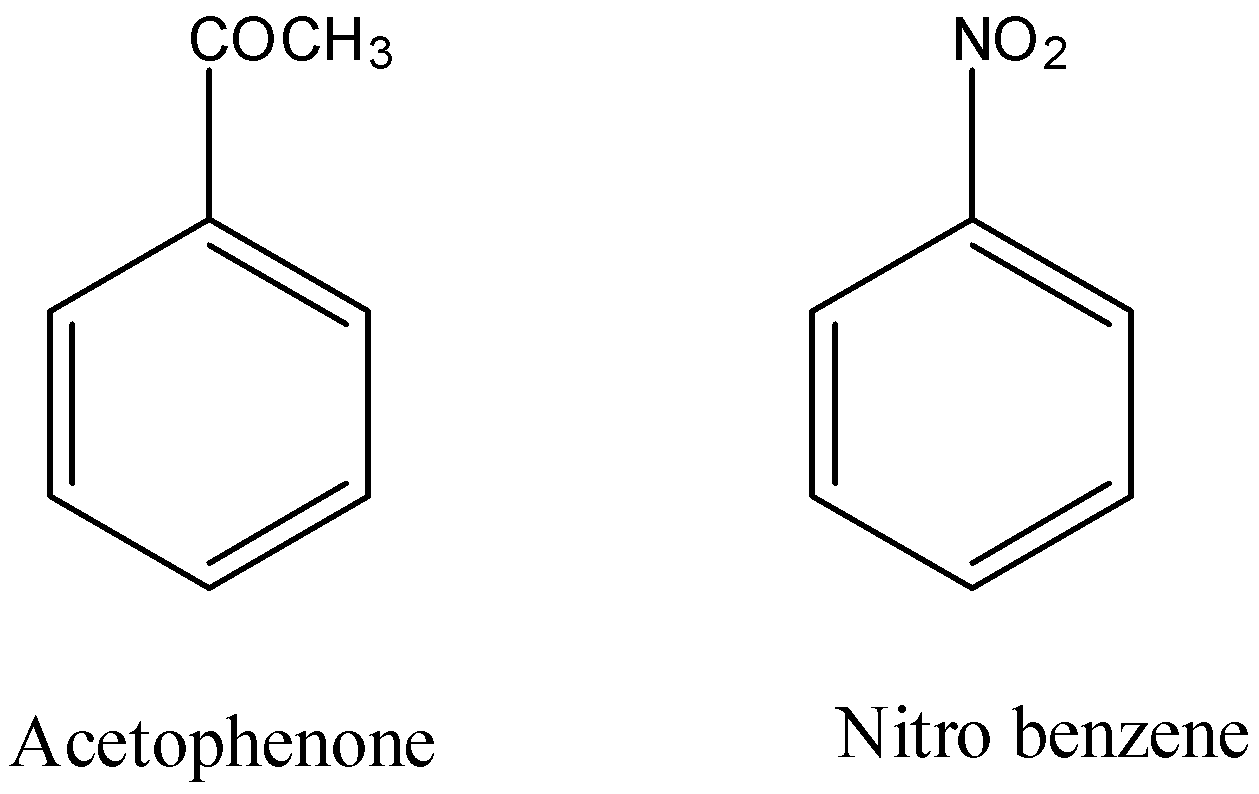
The structure of acetophenone and m-nitrobenzene is as follows.
Complete Solution :
- In the question it is given that to prepare acetophenone from phenol and nitrobenzene from toluene.
(a) Phenol to acetophenone
- The preparation of acetophenone from phenol is a two-step process.
Step -1:
- In the first step phenol is going to convert into benzene in the presence of zinc under heating.
- The chemical reaction of conversion of phenol to benzene is as follows.
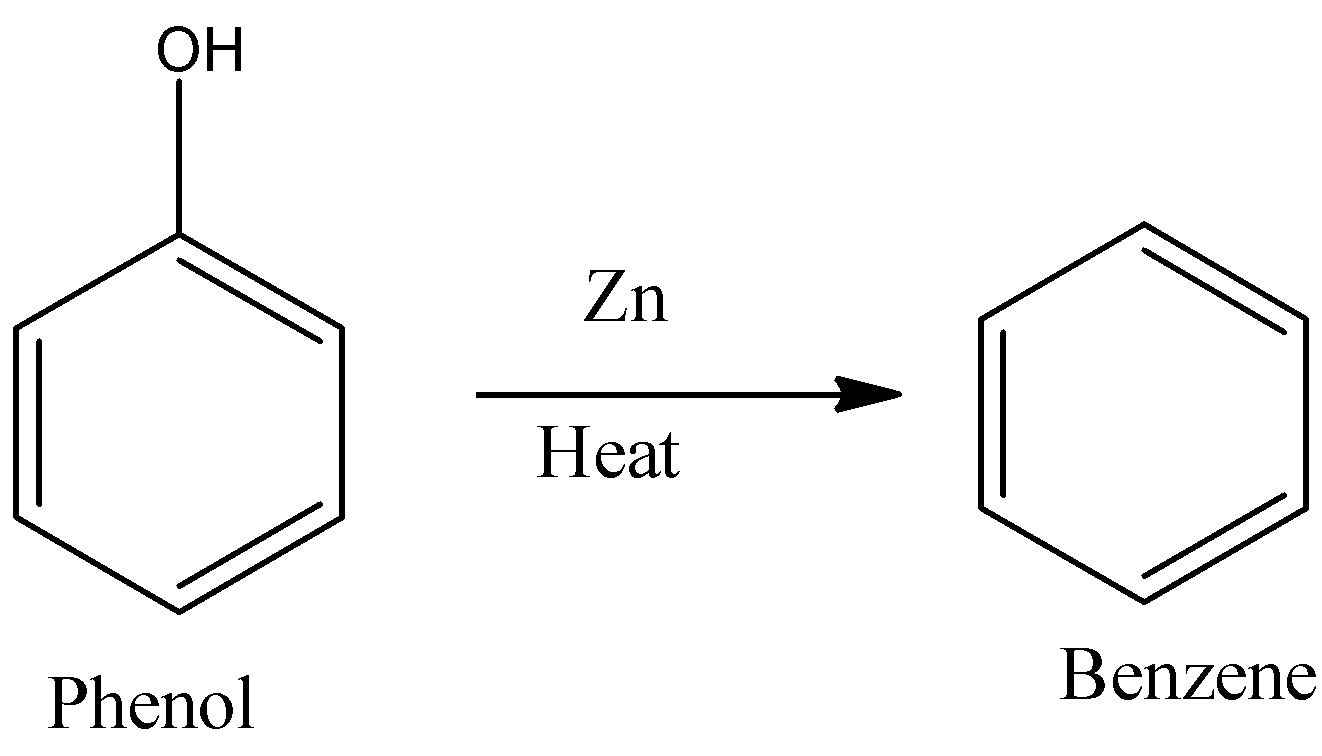
Step -2:
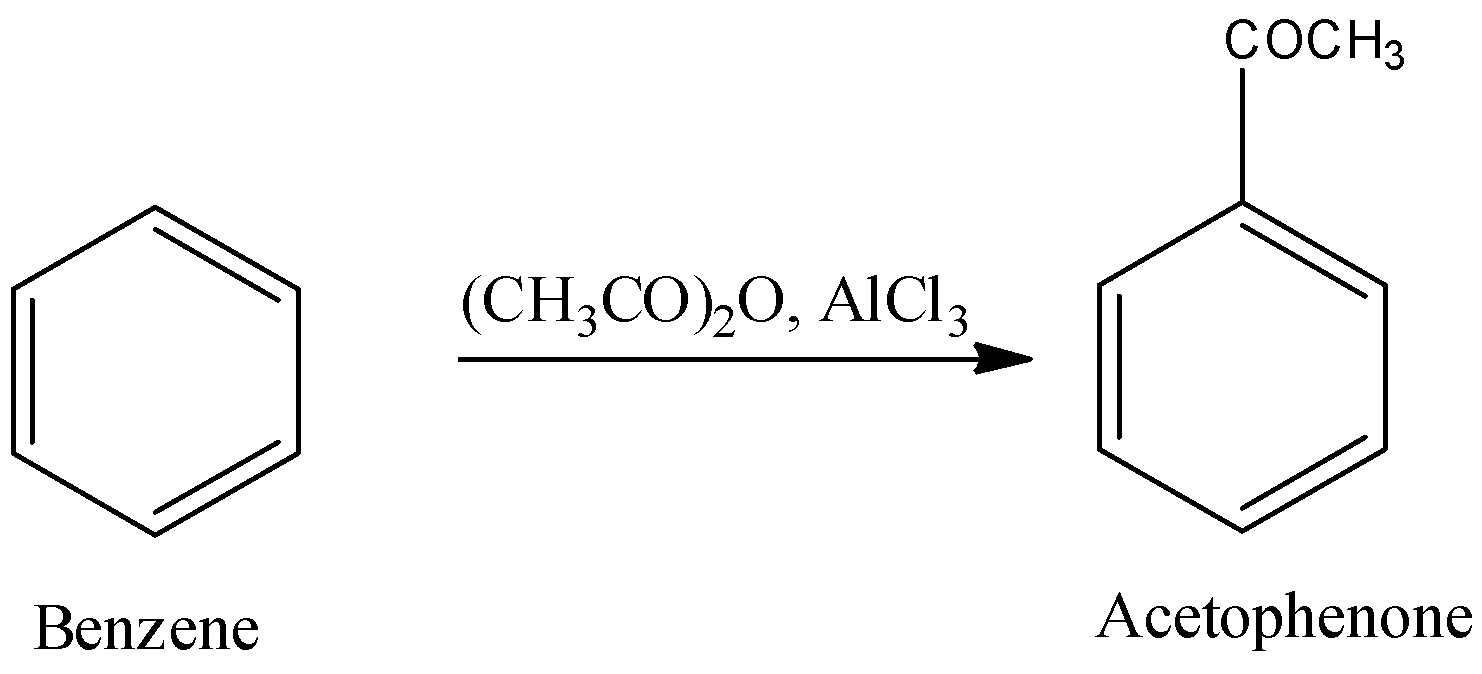
- In the second step the benzene formed in the first step is going to react with acetic anhydride in the presence of aluminium chloride and forms the desired product acetophenone.
- The second step is also called Friedel-Crafts acylation reaction.
- The reaction of benzene with acetic anhydride and aluminium chloride is as follows.
(b) Toluene to m-nitrobenzene
- The preparation of m-nitro benzene from toluene is a three step process.
Step -1:
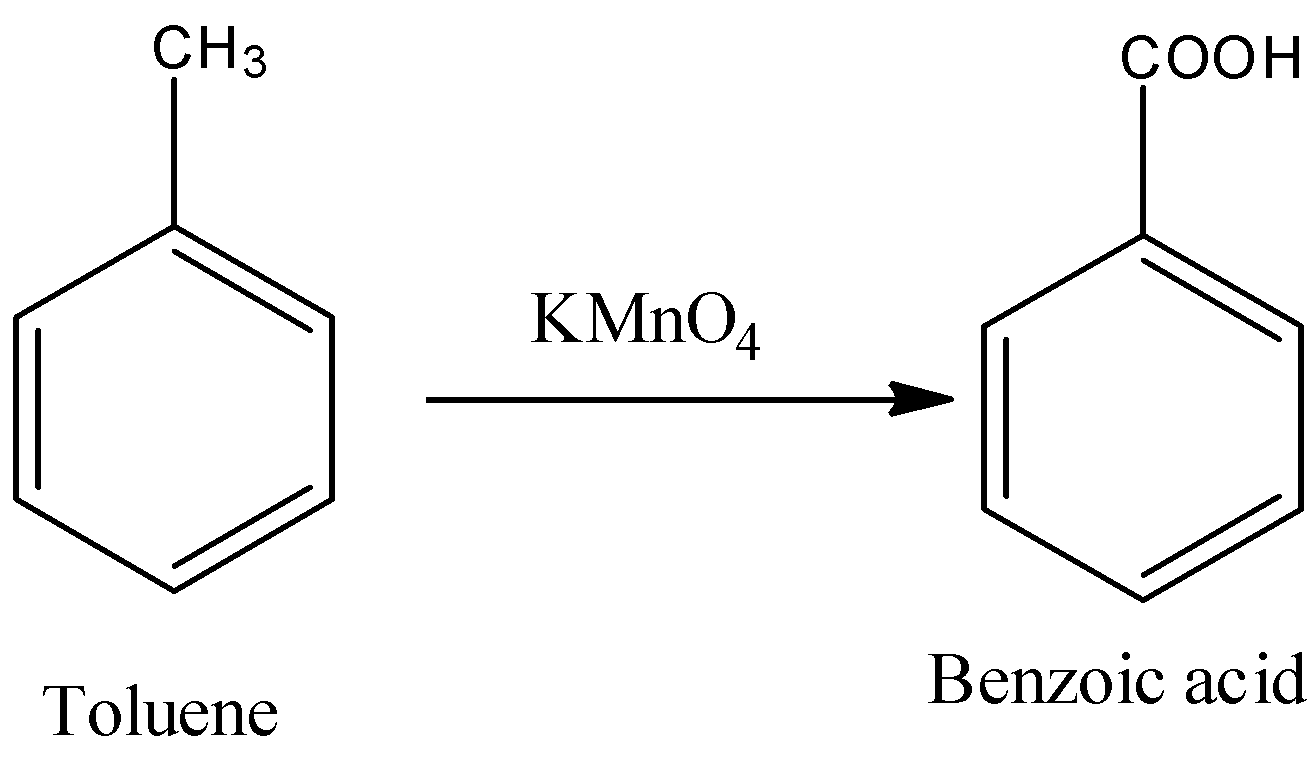
- In the first step toluene is going to convert into benzoic acid in the presence of potassium permanganate.
- The conversion of toluene to benzoic acid is as follows.
Step -2:
- In step-2 the formed benzoic acid in the step-1 reacts with calcium oxide (CaO) and forms benzene as the product.
- The chemical reaction of conversion of benzoic acid to benzene is as follows.
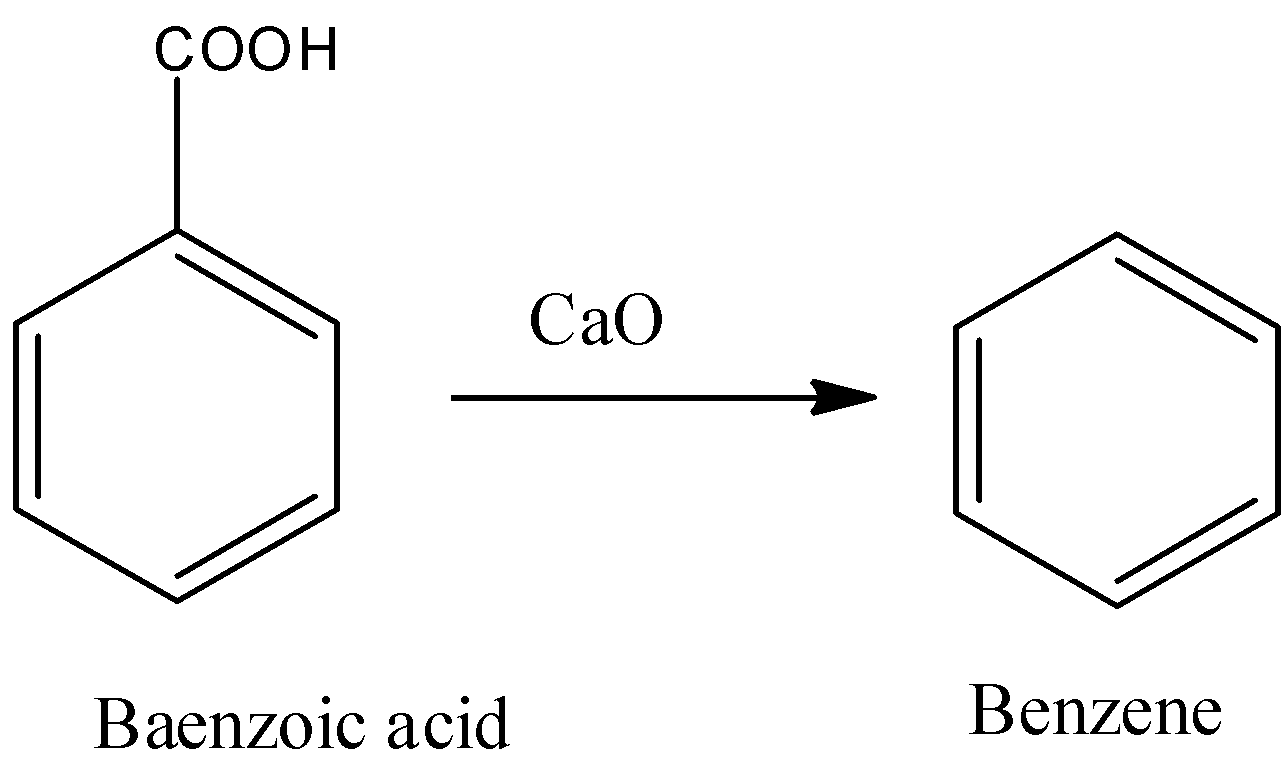
Step -3:
- In step – 3 the formed benzene in the second step reacts with concentrated nitric acid and concentrated sulphuric acid and forms meta-nitrobenzene as the product.
- The chemical reaction of benzene with concentrated nitric and concentrated sulphuric acid is as follows.
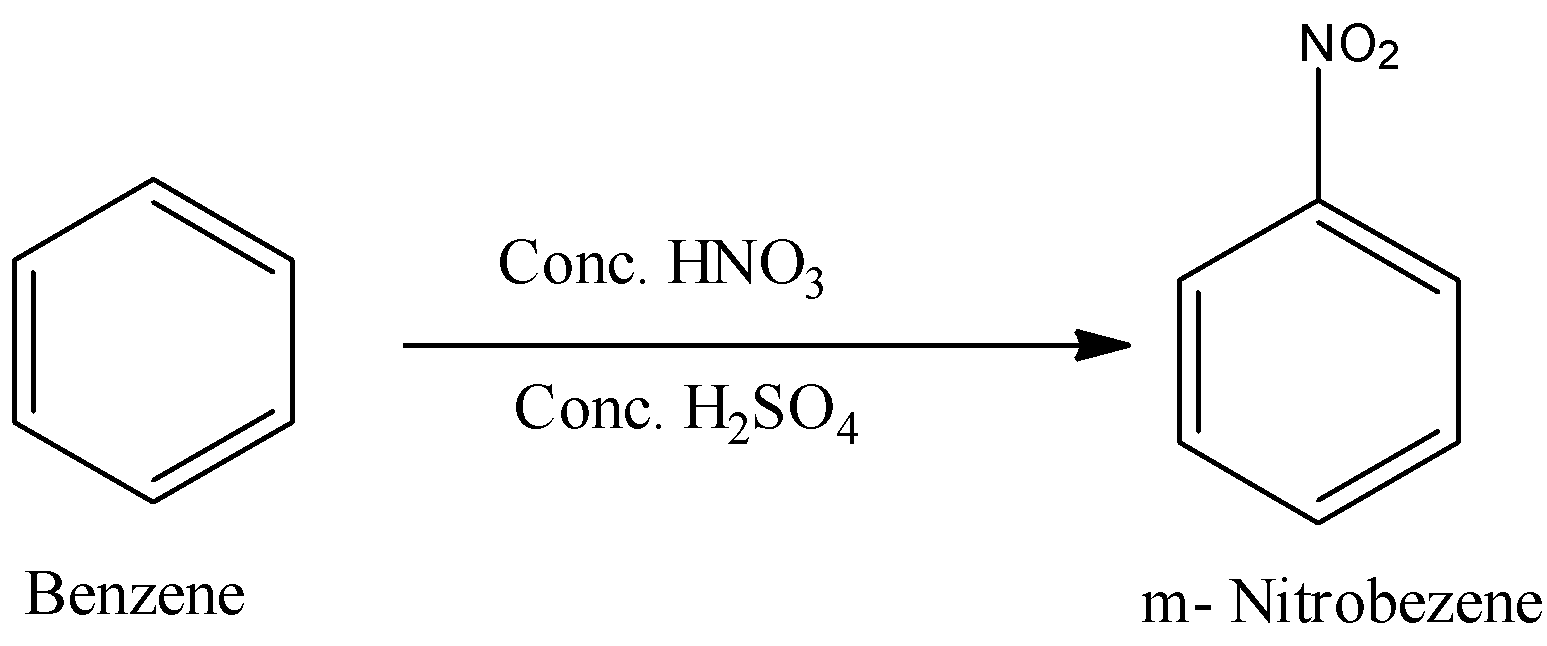
Note: Nitration of benzene is not an easy reaction. To do the nitration of benzene there is a need for strong acid. In the presence of concentrated sulphuric acid nitration can occur on benzene. Nitration of benzene is an example of electrophilic substitution reaction.
The structure of acetophenone and m-nitrobenzene is as follows.

Complete Solution :
- In the question it is given that to prepare acetophenone from phenol and nitrobenzene from toluene.
(a) Phenol to acetophenone
- The preparation of acetophenone from phenol is a two-step process.
Step -1:
- In the first step phenol is going to convert into benzene in the presence of zinc under heating.
- The chemical reaction of conversion of phenol to benzene is as follows.

Step -2:
- In the second step the benzene formed in the first step is going to react with acetic anhydride in the presence of aluminium chloride and forms the desired product acetophenone.
- The second step is also called Friedel-Crafts acylation reaction.
- The reaction of benzene with acetic anhydride and aluminium chloride is as follows.

(b) Toluene to m-nitrobenzene
- The preparation of m-nitro benzene from toluene is a three step process.
Step -1:
- In the first step toluene is going to convert into benzoic acid in the presence of potassium permanganate.
- The conversion of toluene to benzoic acid is as follows.

Step -2:
- In step-2 the formed benzoic acid in the step-1 reacts with calcium oxide (CaO) and forms benzene as the product.
- The chemical reaction of conversion of benzoic acid to benzene is as follows.

Step -3:
- In step – 3 the formed benzene in the second step reacts with concentrated nitric acid and concentrated sulphuric acid and forms meta-nitrobenzene as the product.
- The chemical reaction of benzene with concentrated nitric and concentrated sulphuric acid is as follows.

Note: Nitration of benzene is not an easy reaction. To do the nitration of benzene there is a need for strong acid. In the presence of concentrated sulphuric acid nitration can occur on benzene. Nitration of benzene is an example of electrophilic substitution reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




