
Convert 1 - chloropropane into following:
A.\[Propan - 1 - ol\]
B.\[1 - iodopropane\]
C.Butane nitrile
D.Propyl ethanoate
Answer
571.5k+ views
Hint: Conversion: When a chemical reaction takes place, the process of converting takes place and some potion disappears and is converted to other products. The conversion of $1 - chloropropane$ into the following compounds are simple organic reactions. $1 - chloropropane$ (Also known as n-propyl chloride or$1 - propyl$ $chloride$) is an organic compound. The chemical formula for $1 - chloropropane$ is ${C_3}{H_7}Cl$.
Complete step by step solution:
When the $1 - chloropropane$ is reacted with alcoholic potassium hydroxide,$KOH$, to give the product \[Propan - 1 - ol\], the chemical formula for \[Propan - 1 - ol\] is ${C_3}{H_7}OH$ and potassium chloride,$KCl$, is the byproduct for this reaction.
We can write the chemical equation for this chemical reaction as,
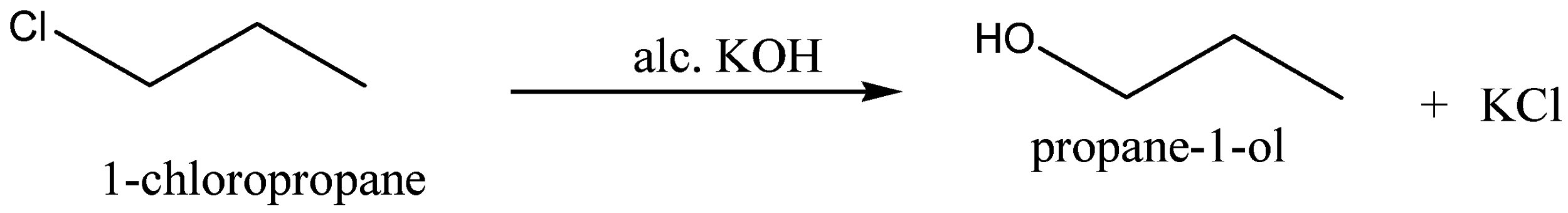
Now we can discuss the conversion of $1 - chloropropane$ into \[1 - iodopropane\].
Now we discuss the conversion of $1 - chloropropane$ into Butane nitrile.
$1 - chloropropane$ is reacted with sodium iodide,$NaI$, to give the corresponding product \[1 - iodopropane\], (chemical formula: ${C_3}{H_7}I$) and also give sodium chloride,$NaCl$, as byproduct.
We can write the chemical equation for this chemical reaction as,
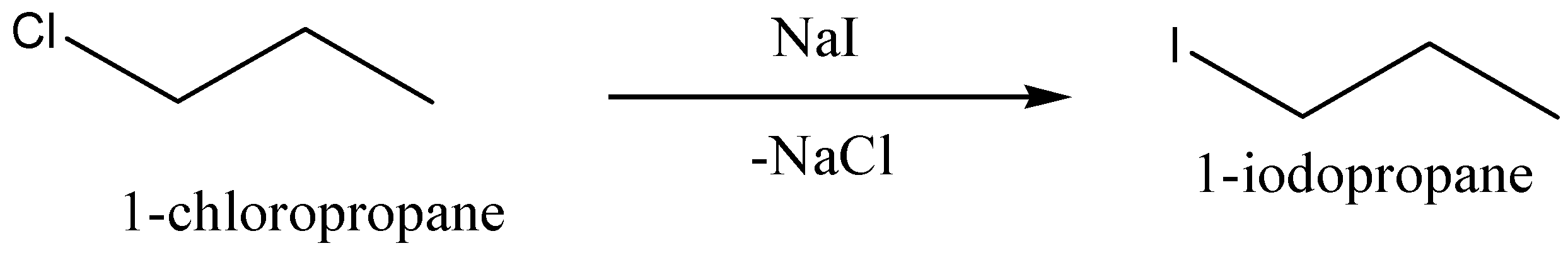
Now we can discuss the reaction of $1 - chloropropane$ with potassium cyanide,$KCN$, to give butane nitrile (chemical formula: ${C_4}{H_7}N$). And potassium chloride,$KCl,$ is eliminated.
We can write the chemical equation for this chemical reaction as,
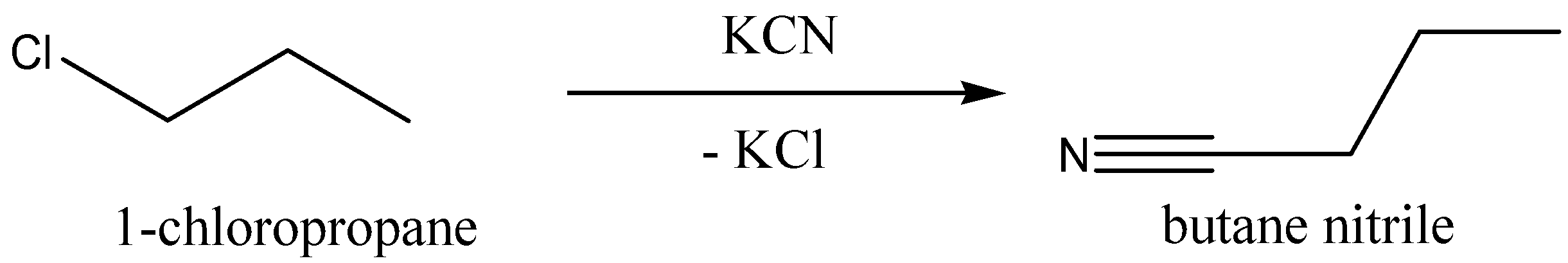
Now we can discuss the conversion of $1 - chloropropane$ into Propyl ethanoate.
When the $1 - chloropropane$ is reacted with alcoholic potassium hydroxide,$KOH$, to give the product \[Propan - 1 - ol\], the chemical formula for \[Propan - 1 - ol\] is ${C_3}{H_7}OH$. And potassium chloride,$KCl$, is the byproduct for this reaction. Then \[Propan - 1 - ol\] is treated with propionic acid, chemical formula-${C_3}{H_6}{O_2}$, to give the corresponding product Propyl ethanoate (chemical formula:${C_5}{H_{10}}{O_2}$), and eliminates water molecule.
We can write the chemical reaction for Conversion of $1 - chloropropane$ into Propyl ethanoate as,
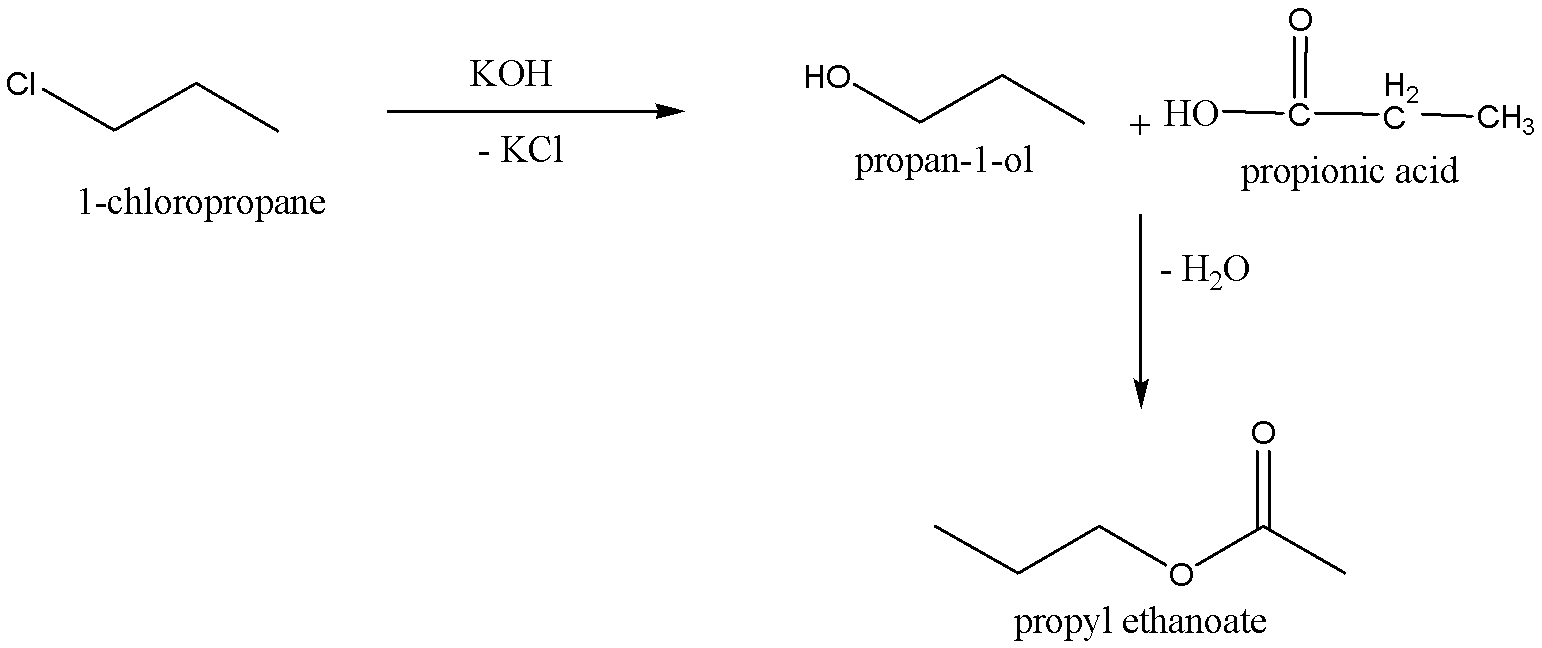
Therefore, the option A and B is correct.
Note: All the above three conversions (options a, b and d) contain the same carbon atoms on both sides, reactant sides and product sides. But in option (c), Conversion of $1 - chloropropane$into Butane nitrile, that butane nitrile containing one carbon atom more than the reactant,$1 - chloropropane$.
$1 - chloropropane$ is used in pharmaceutical intermediate for \[n - propylamine\] and solvent for making other chemicals which is also used in pesticides.
Complete step by step solution:
When the $1 - chloropropane$ is reacted with alcoholic potassium hydroxide,$KOH$, to give the product \[Propan - 1 - ol\], the chemical formula for \[Propan - 1 - ol\] is ${C_3}{H_7}OH$ and potassium chloride,$KCl$, is the byproduct for this reaction.
We can write the chemical equation for this chemical reaction as,

Now we can discuss the conversion of $1 - chloropropane$ into \[1 - iodopropane\].
Now we discuss the conversion of $1 - chloropropane$ into Butane nitrile.
$1 - chloropropane$ is reacted with sodium iodide,$NaI$, to give the corresponding product \[1 - iodopropane\], (chemical formula: ${C_3}{H_7}I$) and also give sodium chloride,$NaCl$, as byproduct.
We can write the chemical equation for this chemical reaction as,
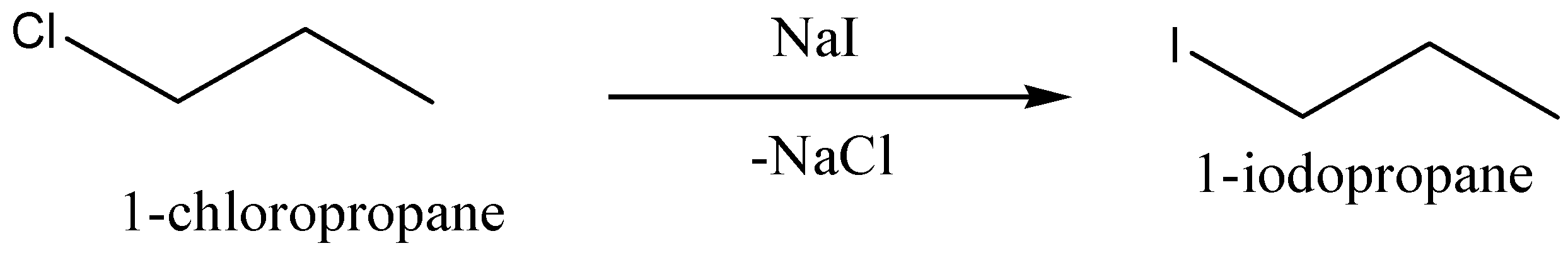
Now we can discuss the reaction of $1 - chloropropane$ with potassium cyanide,$KCN$, to give butane nitrile (chemical formula: ${C_4}{H_7}N$). And potassium chloride,$KCl,$ is eliminated.
We can write the chemical equation for this chemical reaction as,

Now we can discuss the conversion of $1 - chloropropane$ into Propyl ethanoate.
When the $1 - chloropropane$ is reacted with alcoholic potassium hydroxide,$KOH$, to give the product \[Propan - 1 - ol\], the chemical formula for \[Propan - 1 - ol\] is ${C_3}{H_7}OH$. And potassium chloride,$KCl$, is the byproduct for this reaction. Then \[Propan - 1 - ol\] is treated with propionic acid, chemical formula-${C_3}{H_6}{O_2}$, to give the corresponding product Propyl ethanoate (chemical formula:${C_5}{H_{10}}{O_2}$), and eliminates water molecule.
We can write the chemical reaction for Conversion of $1 - chloropropane$ into Propyl ethanoate as,

Therefore, the option A and B is correct.
Note: All the above three conversions (options a, b and d) contain the same carbon atoms on both sides, reactant sides and product sides. But in option (c), Conversion of $1 - chloropropane$into Butane nitrile, that butane nitrile containing one carbon atom more than the reactant,$1 - chloropropane$.
$1 - chloropropane$ is used in pharmaceutical intermediate for \[n - propylamine\] and solvent for making other chemicals which is also used in pesticides.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




