
Consider the given plots for a reaction obeying Arrhenius equation \[\left( {{0^ \circ } < T < {{300}^ \circ }C} \right):\] ( ${K_a}$ and ${E_a}$ are rate constant and activation energy, respectively). Choose the correct option.
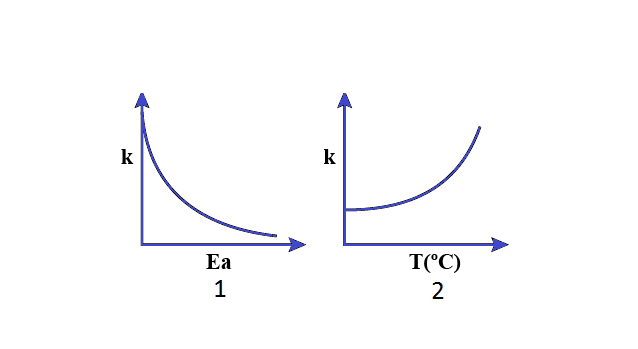
$A.$ Both $1$ and $2$ are wrong
$B.$ $1$ is wrong but $2$ is right
$C.$ Both $1$ and \[2\] are correct
$D.$ $1$ is right but $2$ is wrong

Answer
562.8k+ views
Hint: Before discussing the given plot we should have an idea about the Arrhenius equation ( The Arrhenius equation is a formula for the temperature dependence of reaction rates). This equation was proposed by Svante Arrhenius.
Complete solution:
The given question can be solved if we find the relation between rate constant, Activation energy and temperature. Now find a relation between these quantities.
Here is the Arrhenius equation,
$k = A{e^{ - {E_a}/RT}}$
Where $A$ is the Arrhenius factor or we can also say frequency factor, $R$ is a gas constant, $T$ is a temperature and ${E_a}$ is the activation energy. Activation energy is measured in joule per mole.
From the above equation it has been found that increasing in temperature or decreasing the activation energy will result in an increase in the rate of the reaction and an exponential increase in rate constant.
Since, we have learned from Arrhenius equation when we increase activation energy $\left( {{E_a}} \right)$ , rate constant $\left( k \right)$ should decrease and if we increase the temperature, power of exponential increase so rate constant $\left( k \right)$ also increase.
Hence the correct option is (C).
Addition information: Activation energy is defined as the minimum amount of extra energy required by a reacting molecule to get converted into a product.
Note: It is to be noted that from the Arrhenius equation we found the rate constant mainly depends on temperature and activation energy. It has found that for a chemical reaction with increase in temperature by ${10^ \circ }$ , the rate constant is doubled.
Complete solution:
The given question can be solved if we find the relation between rate constant, Activation energy and temperature. Now find a relation between these quantities.
Here is the Arrhenius equation,
$k = A{e^{ - {E_a}/RT}}$
Where $A$ is the Arrhenius factor or we can also say frequency factor, $R$ is a gas constant, $T$ is a temperature and ${E_a}$ is the activation energy. Activation energy is measured in joule per mole.
From the above equation it has been found that increasing in temperature or decreasing the activation energy will result in an increase in the rate of the reaction and an exponential increase in rate constant.
Since, we have learned from Arrhenius equation when we increase activation energy $\left( {{E_a}} \right)$ , rate constant $\left( k \right)$ should decrease and if we increase the temperature, power of exponential increase so rate constant $\left( k \right)$ also increase.
Hence the correct option is (C).
Addition information: Activation energy is defined as the minimum amount of extra energy required by a reacting molecule to get converted into a product.
Note: It is to be noted that from the Arrhenius equation we found the rate constant mainly depends on temperature and activation energy. It has found that for a chemical reaction with increase in temperature by ${10^ \circ }$ , the rate constant is doubled.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




