
Consider the given plot of enthalpy of the following reaction between A and B. $A+B\to C+D$. Identify the incorrect statement.
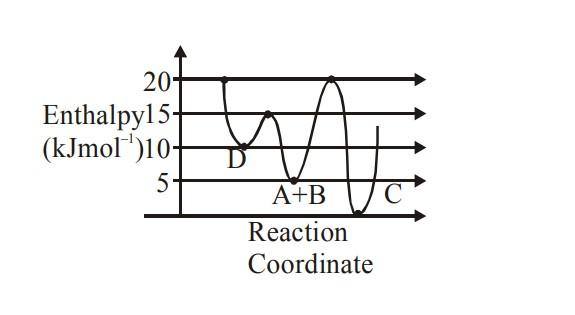
(A) C is the thermodynamically stable product
(B) Formation of A and B from C has highest enthalpy of activation
(C) D is kinetically stable product
(D) Activation enthalpy to form C is 5 $KJmo{{l}^{-1}}$ less than that to form D

Answer
578.1k+ views
Hint: From the plot, we can find the activation enthalpy of C and D. This will allow us to identify the kinetic and thermodynamically stable products. Also, we will be able to identify the product which has more enthalpy of activation and verify the given statements.
Complete step by step solution:
- Let’s start with the concept of enthalpy of activation or activation energy. It can be defined as the minimum amount of extra energy required by a reactant to get converted into a product. Or in other words, it is the minimum amount of energy which is needed to activate the molecules or atoms so that they can undergo a chemical reaction.
- In order to identify the kinetic and thermodynamically stable products in the given reaction, we need to find their enthalpy of activation. From the image, we can find the activation enthalpy of C and D.
The activation enthalpy for C = 20−5= 15$KJmo{{l}^{-1}}$
The activation enthalpy for D =15−5=10 $KJmo{{l}^{-1}}$
- The product with less activation enthalpy will be kinetically favorable and thus D is the kinetically stable product. Similarly, the product with high activation enthalpy will be kinetically unfavorable and thus C is the thermodynamically stable product. Hence the statements (A) and (C) are correct.
- The formation of A and B from C will have an activation enthalpy of 20 $KJmo{{l}^{-1}}$ (20−0) and formation of A and B from D will have an activation enthalpy of 5 $KJmo{{l}^{-1}}$ (15−10). Hence the statement (B) is also correct.
- As we found in the beginning, the activation enthalpy for C is 15 $KJmo{{l}^{-1}}$ and the activation enthalpy for D is 10 $KJmo{{l}^{-1}}$.Thus the activation enthalpy to form C is 5 $KJmo{{l}^{-1}}$ more than that to form .Hence the statement (D) is incorrect.
Therefore, the answer is option (D).
Note: Do not confuse between activation enthalpy and enthalpy of reaction. The enthalpy of activation is the energy required to start the reaction whereas the enthalpy of reaction is equal to the amount of heat absorbed or released during the reaction. The enthalpy of reaction can be obtained by subtracting the enthalpy of reactants from the enthalpy of products
Complete step by step solution:
- Let’s start with the concept of enthalpy of activation or activation energy. It can be defined as the minimum amount of extra energy required by a reactant to get converted into a product. Or in other words, it is the minimum amount of energy which is needed to activate the molecules or atoms so that they can undergo a chemical reaction.
- In order to identify the kinetic and thermodynamically stable products in the given reaction, we need to find their enthalpy of activation. From the image, we can find the activation enthalpy of C and D.
The activation enthalpy for C = 20−5= 15$KJmo{{l}^{-1}}$
The activation enthalpy for D =15−5=10 $KJmo{{l}^{-1}}$
- The product with less activation enthalpy will be kinetically favorable and thus D is the kinetically stable product. Similarly, the product with high activation enthalpy will be kinetically unfavorable and thus C is the thermodynamically stable product. Hence the statements (A) and (C) are correct.
- The formation of A and B from C will have an activation enthalpy of 20 $KJmo{{l}^{-1}}$ (20−0) and formation of A and B from D will have an activation enthalpy of 5 $KJmo{{l}^{-1}}$ (15−10). Hence the statement (B) is also correct.
- As we found in the beginning, the activation enthalpy for C is 15 $KJmo{{l}^{-1}}$ and the activation enthalpy for D is 10 $KJmo{{l}^{-1}}$.Thus the activation enthalpy to form C is 5 $KJmo{{l}^{-1}}$ more than that to form .Hence the statement (D) is incorrect.
Therefore, the answer is option (D).
Note: Do not confuse between activation enthalpy and enthalpy of reaction. The enthalpy of activation is the energy required to start the reaction whereas the enthalpy of reaction is equal to the amount of heat absorbed or released during the reaction. The enthalpy of reaction can be obtained by subtracting the enthalpy of reactants from the enthalpy of products
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




