
Consider the following reaction:
Major Product D is
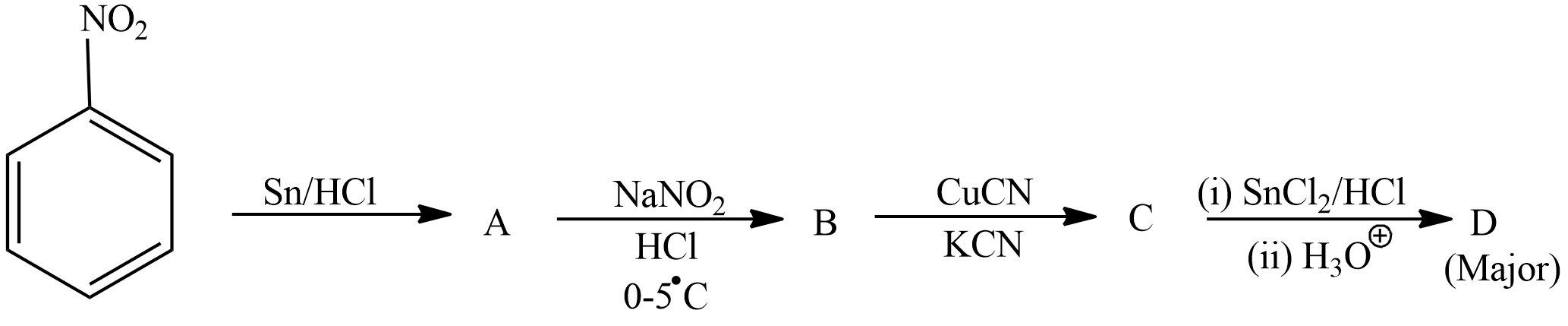
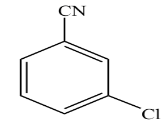
A.
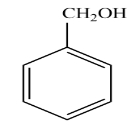
B.
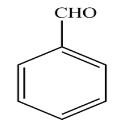
C.
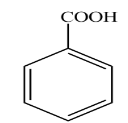
D.





Answer
493.2k+ views
Hint: The given compound is nitrobenzene which is undergoing few reactions to form product A, product B, product C, and product D. Multiple reactions are involved here we will study each reaction and understand the process in order to identify each product formed.
Complete answer: Let’s see the first reaction in which nitrobenzene is treated with Tin in presence of Hydrochloric acid. This is a reduction reaction. The reaction taking place here is written as
In the above reaction nitrobenzene is undergoing reduction in presence of Tin and Hydrochloric acid to form Aniline. So according to the question product A is aniline.
Let’s see the second reaction in which product A is treated with Sodium nitrite and Hydrochloric acid at mild temperature to form product B. The reaction taking place here is written as
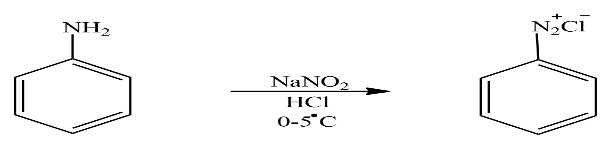
In the above reaction Aniline reacts with sodium nitrite and hydrochloric acid at mild temperature to form Benzene diazonium chloride. It is highly reactive and has low stability. So according to question the product B is Benzene diazonium chloride.
Let’s see the next reaction in which product B is treated with copper cyanide and Potassium cyanide to give product C. It is a substitution reaction. The reaction taking place here is written as
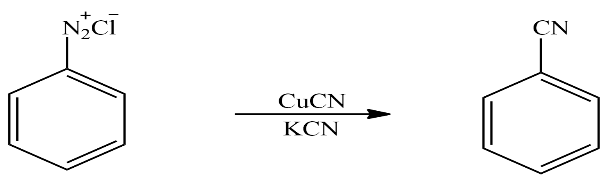
In the above reaction Benzene diazonium chloride reacts with copper cyanide and potassium cyanide to form benzene nitrile, it is a substitution reaction the diazonium group is replaced by cyanide. So according to the question product C is Benzene Nitrile.
let’s see the final reaction in which product C reacts with Stannous chloride and Hydrochloric acid followed by hydrolysis to form product D. the reaction taking place here is written as
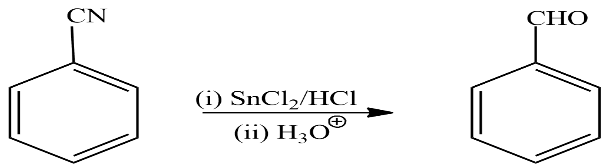
In the above reaction benzene nitrile reacts with stannous chloride and hydrochloric acid followed by hydrolysis to give Benzaldehyde. So according to the question product D is Benzaldehyde.
Note:
The last reaction is known as Stephen reaction used to convert nitriles to aldehydes. It first forms a complex of imine while reacting with stannous chloride and hydrochloric acid and then on hydrolysis it gives aldehyde.
Complete answer: Let’s see the first reaction in which nitrobenzene is treated with Tin in presence of Hydrochloric acid. This is a reduction reaction. The reaction taking place here is written as
In the above reaction nitrobenzene is undergoing reduction in presence of Tin and Hydrochloric acid to form Aniline. So according to the question product A is aniline.
Let’s see the second reaction in which product A is treated with Sodium nitrite and Hydrochloric acid at mild temperature to form product B. The reaction taking place here is written as

In the above reaction Aniline reacts with sodium nitrite and hydrochloric acid at mild temperature to form Benzene diazonium chloride. It is highly reactive and has low stability. So according to question the product B is Benzene diazonium chloride.
Let’s see the next reaction in which product B is treated with copper cyanide and Potassium cyanide to give product C. It is a substitution reaction. The reaction taking place here is written as

In the above reaction Benzene diazonium chloride reacts with copper cyanide and potassium cyanide to form benzene nitrile, it is a substitution reaction the diazonium group is replaced by cyanide. So according to the question product C is Benzene Nitrile.
let’s see the final reaction in which product C reacts with Stannous chloride and Hydrochloric acid followed by hydrolysis to form product D. the reaction taking place here is written as
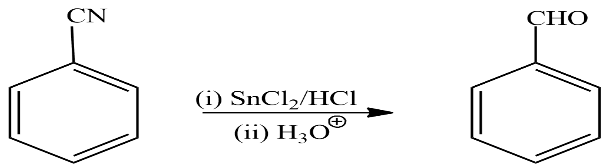
In the above reaction benzene nitrile reacts with stannous chloride and hydrochloric acid followed by hydrolysis to give Benzaldehyde. So according to the question product D is Benzaldehyde.
Note:
The last reaction is known as Stephen reaction used to convert nitriles to aldehydes. It first forms a complex of imine while reacting with stannous chloride and hydrochloric acid and then on hydrolysis it gives aldehyde.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




