
Consider the following compounds. Hyperconjugation occurs in:
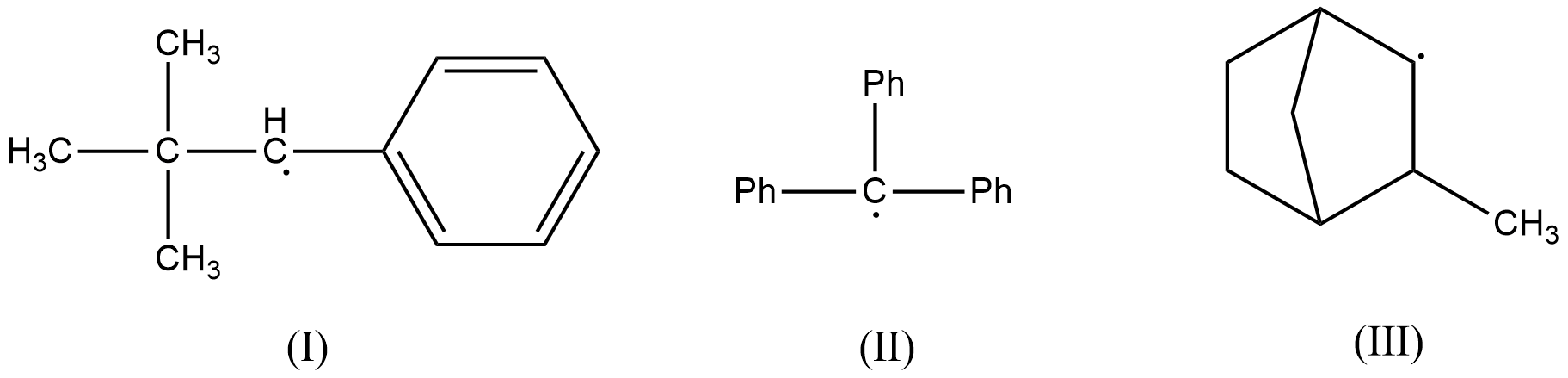
(A) (III) only
(B) I and III
(C) I only
(D) II only

Answer
526.4k+ views
Hint: Think about the concept of hyperconjugation with regards to free radical intermediates. There are three kinds of free radicals given in the question. Find out the type of electronic interaction taking place in each intermediate which is enhancing their stability. Then select the intermediate which is stabilized only by hyperconjugation effect.
Complete answer:
- Hyperconjugation is a stabilizing interaction in which there is interaction of the $\sigma $-electrons of a C-H bond with an adjacent empty non-bonding p-orbital or $\pi $-orbitals to form an extended molecular orbital that increases the stability of the system.
- Hyperconjugation effect is only seen in carbocation and free radical intermediates.
- Now, let’s see an example.
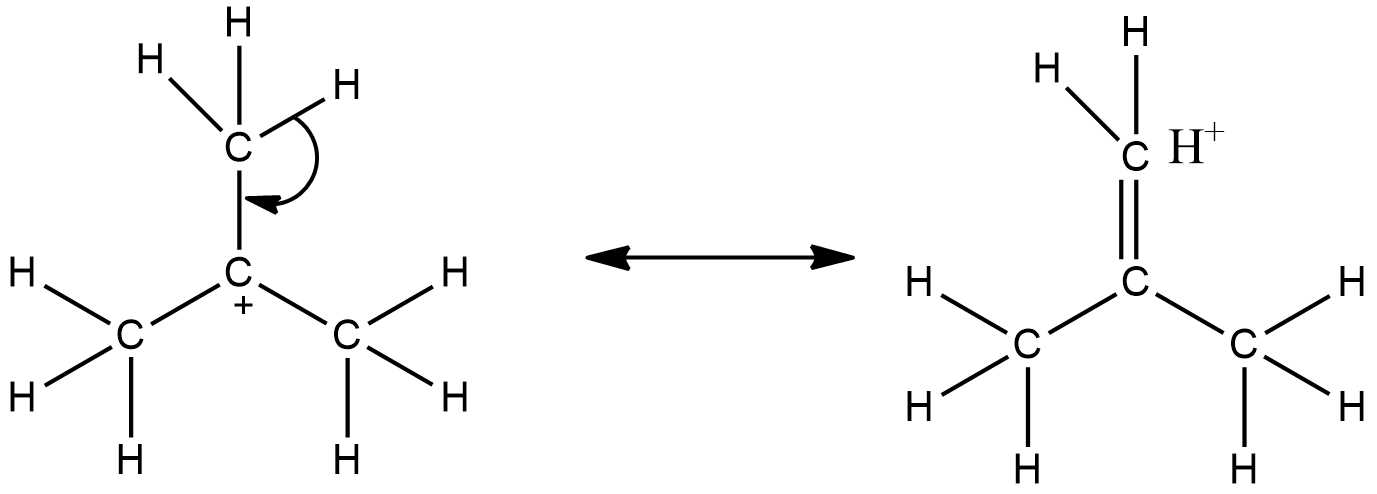
- This shows how a tertiary carbocation is stabilized by hyperconjugation. Here, there are nine possible hyperconjugation structures and so the carbocation is very stable. Similarly, a free radical is also stabilized by hyperconjugation.
- Their main criteria for any ion to get stabilized by hyperconjugation is the presence of an adjacent alkyl group having at least one free hydrogen atom ($\beta $-hydrogen atom) bonded to it.
- Now let’s identify whether the radicals given in the question have a $\beta $-hydrogen atom in them.
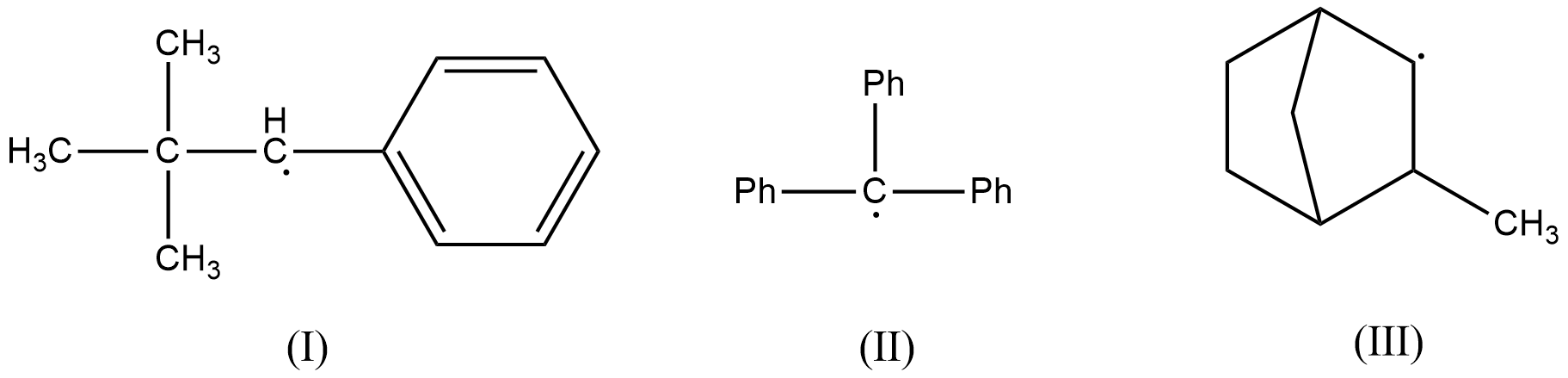
- In the first radical, there is no $\beta $-hydrogen atom present as the carbon radical is bonded to a phenyl ring and a tert-butyl group. Therefore, (I) doesn’t get stabilized by hyperconjugation. This radical is resonance stabilized.
- In the second radical also, there is no $\beta $-hydrogen atom present as the carbon radical is bonded to three phenyl rings. This radical is also resonance stabilized.
- In the third radical, there is a presence of two $\beta $-hydrogen atoms and therefore, this radical is stabilized by hyperconjugation effect.
- Therefore, hyperconjugation occurs in (III) only.
Therefore, the answer is option (A).
Note:
Remember, hyperconjugation effect is a stabilizing interaction which is only possible because of $\beta $-hydrogen atoms present in the structure. Hyperconjugation effects can stabilize carbocation and free radical intermediates only. If phenyl groups are bonded to positively charged carbon then it is resonance stabilized.
Complete answer:
- Hyperconjugation is a stabilizing interaction in which there is interaction of the $\sigma $-electrons of a C-H bond with an adjacent empty non-bonding p-orbital or $\pi $-orbitals to form an extended molecular orbital that increases the stability of the system.
- Hyperconjugation effect is only seen in carbocation and free radical intermediates.
- Now, let’s see an example.

- This shows how a tertiary carbocation is stabilized by hyperconjugation. Here, there are nine possible hyperconjugation structures and so the carbocation is very stable. Similarly, a free radical is also stabilized by hyperconjugation.
- Their main criteria for any ion to get stabilized by hyperconjugation is the presence of an adjacent alkyl group having at least one free hydrogen atom ($\beta $-hydrogen atom) bonded to it.
- Now let’s identify whether the radicals given in the question have a $\beta $-hydrogen atom in them.

- In the first radical, there is no $\beta $-hydrogen atom present as the carbon radical is bonded to a phenyl ring and a tert-butyl group. Therefore, (I) doesn’t get stabilized by hyperconjugation. This radical is resonance stabilized.
- In the second radical also, there is no $\beta $-hydrogen atom present as the carbon radical is bonded to three phenyl rings. This radical is also resonance stabilized.
- In the third radical, there is a presence of two $\beta $-hydrogen atoms and therefore, this radical is stabilized by hyperconjugation effect.
- Therefore, hyperconjugation occurs in (III) only.
Therefore, the answer is option (A).
Note:
Remember, hyperconjugation effect is a stabilizing interaction which is only possible because of $\beta $-hydrogen atoms present in the structure. Hyperconjugation effects can stabilize carbocation and free radical intermediates only. If phenyl groups are bonded to positively charged carbon then it is resonance stabilized.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




