
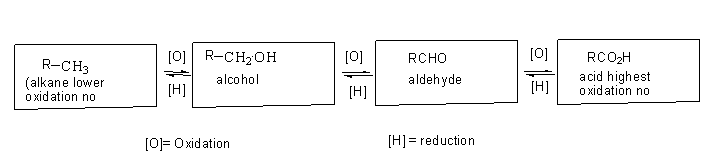
Consider the above sequence and answer the following conversion $R - C{O_2}H \to R - CHO$ can be done by:
A. $LiAl{H_4}$
B. $NaB{H_4}$
C. $DIBAL - H$
D. All of these

Answer
576.9k+ views
Hint:It is an organometallic compound in which the metal atom is bonded to a hydrogen atom and bonded to two isobutyl groups. This compound is a reducing agent and also used to reduce the ester group or a nitrile group to an aldehyde.
Complete step by step answer:
Lithium aluminium hydride $LiAl{H_4}$ is a strong reducing agent used to convert aldehydic groups containing compounds or ketone containing compounds to alcohol.
It donates its hydride to ketone and aldehyde.
Lithium aluminium hydride converts aldehyde to primary alcohol and it converts ketone to a secondary alcohol.
Sodium Borohydride $NaB{H_4}$is a mild reducing agent which is capable of reducing aldehyde and ketones.
Sodium borohydride reduces aldehyde to primary alcohol and it converts ketone to a secondary alcohol.
Diisobutylaluminium hydride is a strong reducing agent that is used to reduce carboxylic acid into an aldehyde. It also converts ester to aldehyde and nitrile to aldehyde. It further does not reduce aldehyde if one more equivalent of reagent is added.
Thus, the conversion of $RC{O_2}H \to RCHO$ is done by using DIBAL-H.
Therefore, the correct option is C.
Additional information: The aldehyde can be converted to the carboxylic acid by oxidation. The oxidizing agent used for the conversion is potassium permanganate in aqueous solution, potassium chromate, hydrogen peroxide, and other oxidizing reagents.
Note:
DIBAL-H can reduce all the compounds in which sodium borohydride reduces therefore ketone and aldehyde can be reduced to primary alcohol and secondary alcohol with the help of DIBAL-H.
Complete step by step answer:
Lithium aluminium hydride $LiAl{H_4}$ is a strong reducing agent used to convert aldehydic groups containing compounds or ketone containing compounds to alcohol.
It donates its hydride to ketone and aldehyde.
Lithium aluminium hydride converts aldehyde to primary alcohol and it converts ketone to a secondary alcohol.
Sodium Borohydride $NaB{H_4}$is a mild reducing agent which is capable of reducing aldehyde and ketones.
Sodium borohydride reduces aldehyde to primary alcohol and it converts ketone to a secondary alcohol.
Diisobutylaluminium hydride is a strong reducing agent that is used to reduce carboxylic acid into an aldehyde. It also converts ester to aldehyde and nitrile to aldehyde. It further does not reduce aldehyde if one more equivalent of reagent is added.
Thus, the conversion of $RC{O_2}H \to RCHO$ is done by using DIBAL-H.
Therefore, the correct option is C.
Additional information: The aldehyde can be converted to the carboxylic acid by oxidation. The oxidizing agent used for the conversion is potassium permanganate in aqueous solution, potassium chromate, hydrogen peroxide, and other oxidizing reagents.
Note:
DIBAL-H can reduce all the compounds in which sodium borohydride reduces therefore ketone and aldehyde can be reduced to primary alcohol and secondary alcohol with the help of DIBAL-H.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




