
What is the configuration of the given molecule?
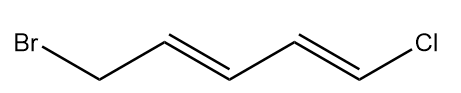
(a) $(1E,\;3Z)$
(b) $(1E,\;3E)$
(c) $(1Z,\;3Z)$
(d) $(1Z,\;3E)$

Answer
509.4k+ views
Hint: The spatial arrangement of atoms in a chiral molecule, and stereochemical description of that atom is known as its absolute configuration. It is determined by CIP rule, according to which the functional group attached to the chiral carbon with higher atomic number will get a greater priority than the functional group with smaller atomic number.
Complete answer:
There are many ways to assign absolute configuration to a molecule. E-Z system is one of the methods according to which, if the groups with same priority are connected to alkene on the same side, then it is said to be Z configuration whereas if the groups with same priority are present on the opposite side of the alkene, then it is termed as E configuration.
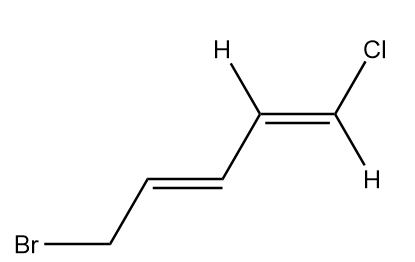
In the given molecule, we double bonds at two positions. Let’s look at the configuration of each double bond separately.
Configuration of double bond at first position:
In the molecule, at first terminal chlorine has higher atomic number than hydrogen, so it will be given higher priority whereas in the second terminal of alkene, the carbon atom has higher atomic number than hydrogen, so it will have greater priority.
Therefore, the priorities given are as follows:
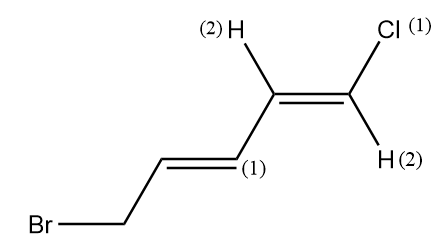
As the groups with the same priority are present at the opposite sides of the alkene, therefore it has E configuration.
Configuration of double bond at third position:
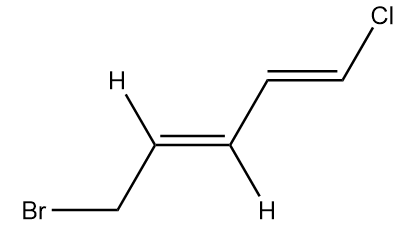
In the molecule, at first the carbon atom has higher atomic number than hydrogen, so it will be given higher priority whereas in the second terminal of alkene, again the carbon atom has higher atomic number than hydrogen, so it will have greater priority.
Therefore, the priorities given are as follows:
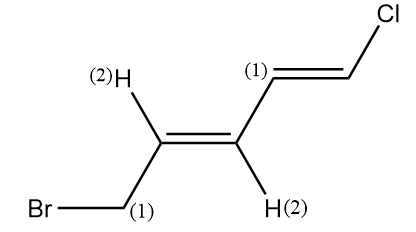
As the groups with the same priority are present at the opposite sides of the alkene, therefore it has E configuration.
Hence, for the given molecule the absolute configuration is $(1E,\,3E)$.
Therefore, the correct answer is option B.
Note:
Other than E-Z configuration, the other absolute configurations which are based on CIP rules are R-S configuration that is an important nomenclature for denoting enantiomers and d-l configuration that is determined by a direction in which an optically active compound rotates a plane of polarized light.
Complete answer:
There are many ways to assign absolute configuration to a molecule. E-Z system is one of the methods according to which, if the groups with same priority are connected to alkene on the same side, then it is said to be Z configuration whereas if the groups with same priority are present on the opposite side of the alkene, then it is termed as E configuration.
In the given molecule, we double bonds at two positions. Let’s look at the configuration of each double bond separately.
Configuration of double bond at first position:

In the molecule, at first terminal chlorine has higher atomic number than hydrogen, so it will be given higher priority whereas in the second terminal of alkene, the carbon atom has higher atomic number than hydrogen, so it will have greater priority.
Therefore, the priorities given are as follows:

As the groups with the same priority are present at the opposite sides of the alkene, therefore it has E configuration.
Configuration of double bond at third position:

In the molecule, at first the carbon atom has higher atomic number than hydrogen, so it will be given higher priority whereas in the second terminal of alkene, again the carbon atom has higher atomic number than hydrogen, so it will have greater priority.
Therefore, the priorities given are as follows:

As the groups with the same priority are present at the opposite sides of the alkene, therefore it has E configuration.
Hence, for the given molecule the absolute configuration is $(1E,\,3E)$.
Therefore, the correct answer is option B.
Note:
Other than E-Z configuration, the other absolute configurations which are based on CIP rules are R-S configuration that is an important nomenclature for denoting enantiomers and d-l configuration that is determined by a direction in which an optically active compound rotates a plane of polarized light.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




