
Compound \[{\rm{P}}\] and \[{\rm{R}}\] upon ozonolysis produce \[{\rm{Q}}\] and \[{\rm{S}}\], respectively. The molecular formula of \[{\rm{Q}}\] and \[{\rm{S}}\] is \[{{\rm{C}}_{\rm{8}}}{{\rm{H}}_{\rm{8}}}{\rm{O}}\]. \[{\rm{Q}}\] undergoes Cannizzaro reaction but not Haloform reaction, whereas \[{\rm{S}}\] undergoes Haloform reaction but not Cannizzaro reaction.
The option(s) with suitable combination of \[{\rm{P}}\]and \[{\rm{R}}\] respectively, is (are):
A.
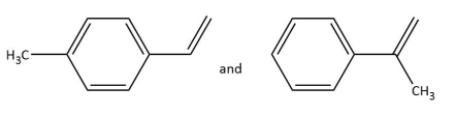
B.
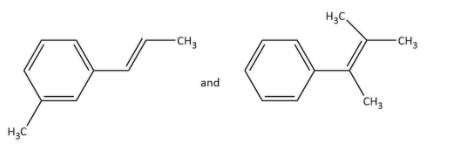
C.
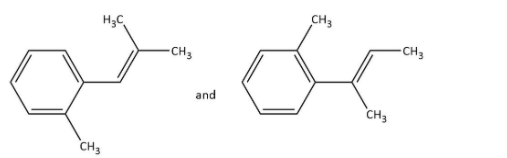
D.
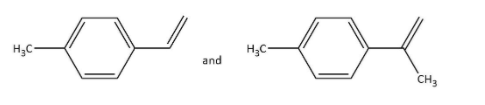




Answer
577.5k+ views
Hint: As we know that, ozonolysis is a method of oxidation of alkene to corresponding aldehyde or ketone in the presence of reducing agent. The products from this reaction can further disproportionate into corresponding alcohol and acid.
Complete step by step answer
Ozonolysis is a method to produce ketone and aldehyde in the presence of reducing agent but when it undergoes in the presence of peroxide, aldehydes change into acid.
Cannizzaro reaction- It is the reaction of two aldehydes which do not have alpha hydrogen. When the reaction occurs, it undergoes disproportionation and forms corresponding alcohol and acid.
Haloform reaction- it is the reaction in which methyl group is present such as acetaldehyde, methyl ketone, primary alcohols (methanol, propane-\[{\rm{2 - ol}}\], butane-\[{\rm{2 - ol}}\]etc.). when the reaction undergoes in the presence of bromine, iodine molecule and a base then it produces corresponding haloform.
Let’s move towards options-
Ozonolysis of \[{\rm{P}}\] in option (A), gives as-
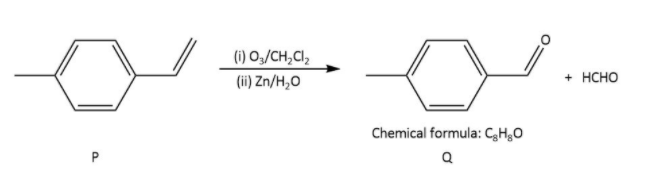
In which \[{\rm{Q}}\] undergoes Cannizzaro reaction but not Haloform reaction because it does not contain any alpha hydrogen.
Ozonolysis of \[{\rm{R}}\] in option (A), gives as-
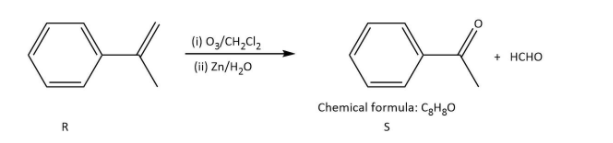
In which \[{\rm{S}}\] undergoes a Haloform reaction but not Cannizzaro reaction as it has a methyl group.
Ozonolysis of \[{\rm{P}}\] in option (B), gives as-
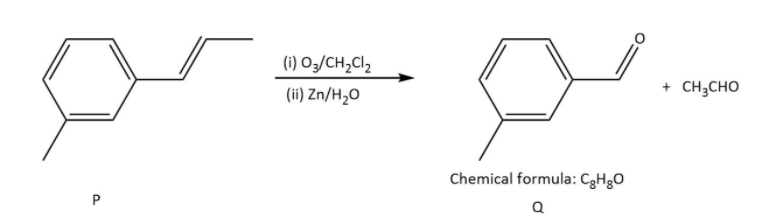
In which \[{\rm{Q}}\] undergoes Cannizzaro reaction but not Haloform reaction because it does not contain any alpha hydrogen.
Ozonolysis of \[{\rm{R}}\] in option (B), gives as-
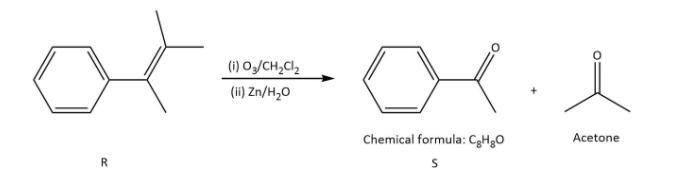
In which \[{\rm{S}}\] undergoes a Haloform reaction but not a Cannizzaro reaction as it has a methyl group.
Rest of the options on ozonolysis do not give products with the given molecular formula.
Therefore, options (A) and (B) are correct options.
Note:
Cannizzaro reaction and Haloform reactions are also used to distinguish between the compounds which have alpha hydrogen or methyl group or not.
Complete step by step answer
Ozonolysis is a method to produce ketone and aldehyde in the presence of reducing agent but when it undergoes in the presence of peroxide, aldehydes change into acid.
Cannizzaro reaction- It is the reaction of two aldehydes which do not have alpha hydrogen. When the reaction occurs, it undergoes disproportionation and forms corresponding alcohol and acid.
Haloform reaction- it is the reaction in which methyl group is present such as acetaldehyde, methyl ketone, primary alcohols (methanol, propane-\[{\rm{2 - ol}}\], butane-\[{\rm{2 - ol}}\]etc.). when the reaction undergoes in the presence of bromine, iodine molecule and a base then it produces corresponding haloform.
Let’s move towards options-
Ozonolysis of \[{\rm{P}}\] in option (A), gives as-

In which \[{\rm{Q}}\] undergoes Cannizzaro reaction but not Haloform reaction because it does not contain any alpha hydrogen.
Ozonolysis of \[{\rm{R}}\] in option (A), gives as-

In which \[{\rm{S}}\] undergoes a Haloform reaction but not Cannizzaro reaction as it has a methyl group.
Ozonolysis of \[{\rm{P}}\] in option (B), gives as-

In which \[{\rm{Q}}\] undergoes Cannizzaro reaction but not Haloform reaction because it does not contain any alpha hydrogen.
Ozonolysis of \[{\rm{R}}\] in option (B), gives as-

In which \[{\rm{S}}\] undergoes a Haloform reaction but not a Cannizzaro reaction as it has a methyl group.
Rest of the options on ozonolysis do not give products with the given molecular formula.
Therefore, options (A) and (B) are correct options.
Note:
Cannizzaro reaction and Haloform reactions are also used to distinguish between the compounds which have alpha hydrogen or methyl group or not.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




