
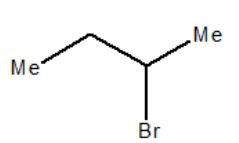
Compound \[\left( {\operatorname{Sec} - {\text{Butyl}} - {\text{bromide}}} \right)\] undergoes alkaline hydrolysis by:
A.\[{{\text{S}}_{\text{N}}}1\]
B.\[{{\text{S}}_{\text{N}}}2\]
C.Both A and B
D.None

Answer
544.8k+ views
Hint: \[{{\text{S}}_{\text{N}}}1\] involves formation of carbocation and the species which can form stable carbocation are more likely to \[{{\text{S}}_{\text{N}}}1\] mechanism. In the case of \[{{\text{S}}_{\text{N}}}2\] mechanism, species with least steric hindrance are more likely to follow \[{{\text{S}}_{\text{N}}}2\] path.
Complete step-by-step answer:In case of \[{{\text{S}}_{\text{N}}}1\] , it is a nucleophilic substitution unimolecular reaction. It is a two step reaction, first step involves formation of carbocation and it is the rate determining step and in second step nucleophile attacks on carbocation intermediate. Characteristics of \[{{\text{S}}_{\text{N}}}1\] reactions are unimolecular which means its rate determining step, formation of carbocation is of first order reaction. The species which forms stable carbocation in the first step is more likely to show \[{{\text{S}}_{\text{N}}}1\] mechanism. Rearrangement of carbocation can take place but this will not affect the rate. In this mechanism, polar protic solvent like water, acetic acid, methanol, and ethanol are used. This mechanism is exothermic and prefers to be performed at low temperature.
In case of \[{{\text{S}}_{\text{N}}}2\] , it is a nucleophilic substitution bimolecular reaction. It is a one step reaction which involves attack of nucleophile on substrate; this step is of second order reaction and is a rate determining step. The steric hindrance affects the rate of this reaction by making difficulty in attack of nucleophile on the species. There is no formation of carbocation and transition state cannot be isolated from the reaction. The nucleophile donate its electrons in an anti bonding orbital of carbon. The solvent used is polar aprotic like DMSO and DMG. The reaction is exothermic and prefers to perform at low temperature.
As in case of given reactant \[\left( {\operatorname{Sec} - {\text{Butyl}} - {\text{bromide}}} \right)\] , due to the steric hindrance by methyl and ethyl group of carbon, it is more likely to follow \[{{\text{S}}_{\text{N}}}1\] mechanism as due to steric hindrance b these group, it will cause difficulty in attack of nucleophile on carbon and also it is a secondary halide which can forms a stable carbocation intermediate which is favorable for \[{{\text{S}}_{\text{N}}}1\] mechanism.
Thus, the correct option is A.
Note: In \[{{\text{S}}_{\text{N}}}1\] mechanism, the nucleophile can attack on both the sides namely front and back. So the product formed is a mixture of both the stereoisomers, also known as a racemic mixture. But in case of \[{{\text{S}}_{\text{N}}}2\] , only backside attack, Walden inversion.
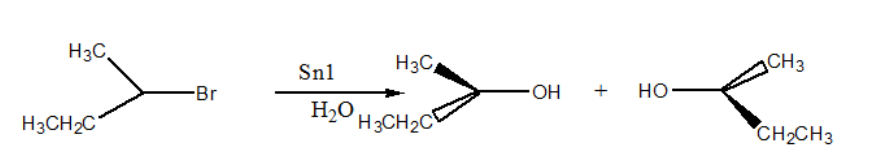
Complete step-by-step answer:In case of \[{{\text{S}}_{\text{N}}}1\] , it is a nucleophilic substitution unimolecular reaction. It is a two step reaction, first step involves formation of carbocation and it is the rate determining step and in second step nucleophile attacks on carbocation intermediate. Characteristics of \[{{\text{S}}_{\text{N}}}1\] reactions are unimolecular which means its rate determining step, formation of carbocation is of first order reaction. The species which forms stable carbocation in the first step is more likely to show \[{{\text{S}}_{\text{N}}}1\] mechanism. Rearrangement of carbocation can take place but this will not affect the rate. In this mechanism, polar protic solvent like water, acetic acid, methanol, and ethanol are used. This mechanism is exothermic and prefers to be performed at low temperature.
In case of \[{{\text{S}}_{\text{N}}}2\] , it is a nucleophilic substitution bimolecular reaction. It is a one step reaction which involves attack of nucleophile on substrate; this step is of second order reaction and is a rate determining step. The steric hindrance affects the rate of this reaction by making difficulty in attack of nucleophile on the species. There is no formation of carbocation and transition state cannot be isolated from the reaction. The nucleophile donate its electrons in an anti bonding orbital of carbon. The solvent used is polar aprotic like DMSO and DMG. The reaction is exothermic and prefers to perform at low temperature.
As in case of given reactant \[\left( {\operatorname{Sec} - {\text{Butyl}} - {\text{bromide}}} \right)\] , due to the steric hindrance by methyl and ethyl group of carbon, it is more likely to follow \[{{\text{S}}_{\text{N}}}1\] mechanism as due to steric hindrance b these group, it will cause difficulty in attack of nucleophile on carbon and also it is a secondary halide which can forms a stable carbocation intermediate which is favorable for \[{{\text{S}}_{\text{N}}}1\] mechanism.
Thus, the correct option is A.
Note: In \[{{\text{S}}_{\text{N}}}1\] mechanism, the nucleophile can attack on both the sides namely front and back. So the product formed is a mixture of both the stereoisomers, also known as a racemic mixture. But in case of \[{{\text{S}}_{\text{N}}}2\] , only backside attack, Walden inversion.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




