
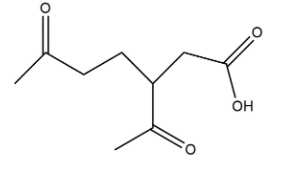
Compound L has the molecular formula${{C}_{10}}{{H}_{16}}$. A sample of L reacted with an excess of hot, concentrated, acidified potassium manganate (VII). Compound M is produced. What would be the structure of L.
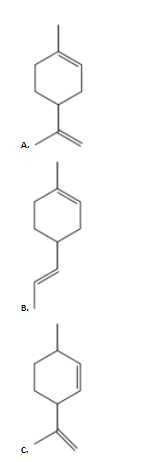


Answer
576.6k+ views
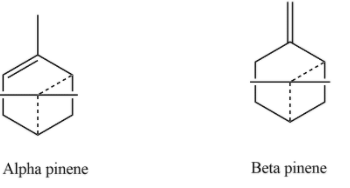
Hint: ${{C}_{10}}{{H}_{16}}$ is known by many names like camphene, carene, limonene etc. but the most commonly known is pinenes this type of compounds are kept in the category of pinenes which further categorized into $\alpha -pinene$ and $\beta -pinene$.
Complete Step by step solution: Pinene is generally represented by the molecular formula ${{C}_{10}}{{H}_{16}}$ which is generally a bicyclic monoterpene chemical compound. There are two natural structural isomers of pentene given by $\alpha -pinene$ and $\beta -pinene$. As the name of isomers of pinene suggests that both forms are important constituents of pine resin i.e. they are also found in the resins of many other conifers as well as in non-coniferous plants for example camphorweed and big sagebrush. Both isomers of pinene constitute the major component of turpentine and are used by many insects in their chemical communication system. $\alpha -pinene$ and $\beta -pinene$ can be shown as:
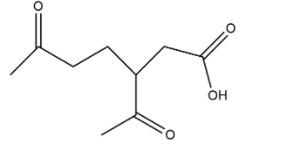
Sample L as discussed is generally a pinene which when react with acidified potassium manganate gives
Hence we can say that L is shown as:
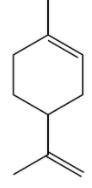
alpha pinene; Option A is the correct answer.
Note: These compounds are used in the chemical industry and oxidation reaction of pinene with some catalysts gives many compounds like perfumes and artificial odorants. Important oxidation product is verbenone, along with pinene oxide, verbenol, and verbenyl hydroperoxide. Pinenes are the primary constituents of turpentine. Pinene dimers seem to have heating values comparable to jet fuel.
Complete Step by step solution: Pinene is generally represented by the molecular formula ${{C}_{10}}{{H}_{16}}$ which is generally a bicyclic monoterpene chemical compound. There are two natural structural isomers of pentene given by $\alpha -pinene$ and $\beta -pinene$. As the name of isomers of pinene suggests that both forms are important constituents of pine resin i.e. they are also found in the resins of many other conifers as well as in non-coniferous plants for example camphorweed and big sagebrush. Both isomers of pinene constitute the major component of turpentine and are used by many insects in their chemical communication system. $\alpha -pinene$ and $\beta -pinene$ can be shown as:

Sample L as discussed is generally a pinene which when react with acidified potassium manganate gives

Hence we can say that L is shown as:

alpha pinene; Option A is the correct answer.
Note: These compounds are used in the chemical industry and oxidation reaction of pinene with some catalysts gives many compounds like perfumes and artificial odorants. Important oxidation product is verbenone, along with pinene oxide, verbenol, and verbenyl hydroperoxide. Pinenes are the primary constituents of turpentine. Pinene dimers seem to have heating values comparable to jet fuel.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




